The
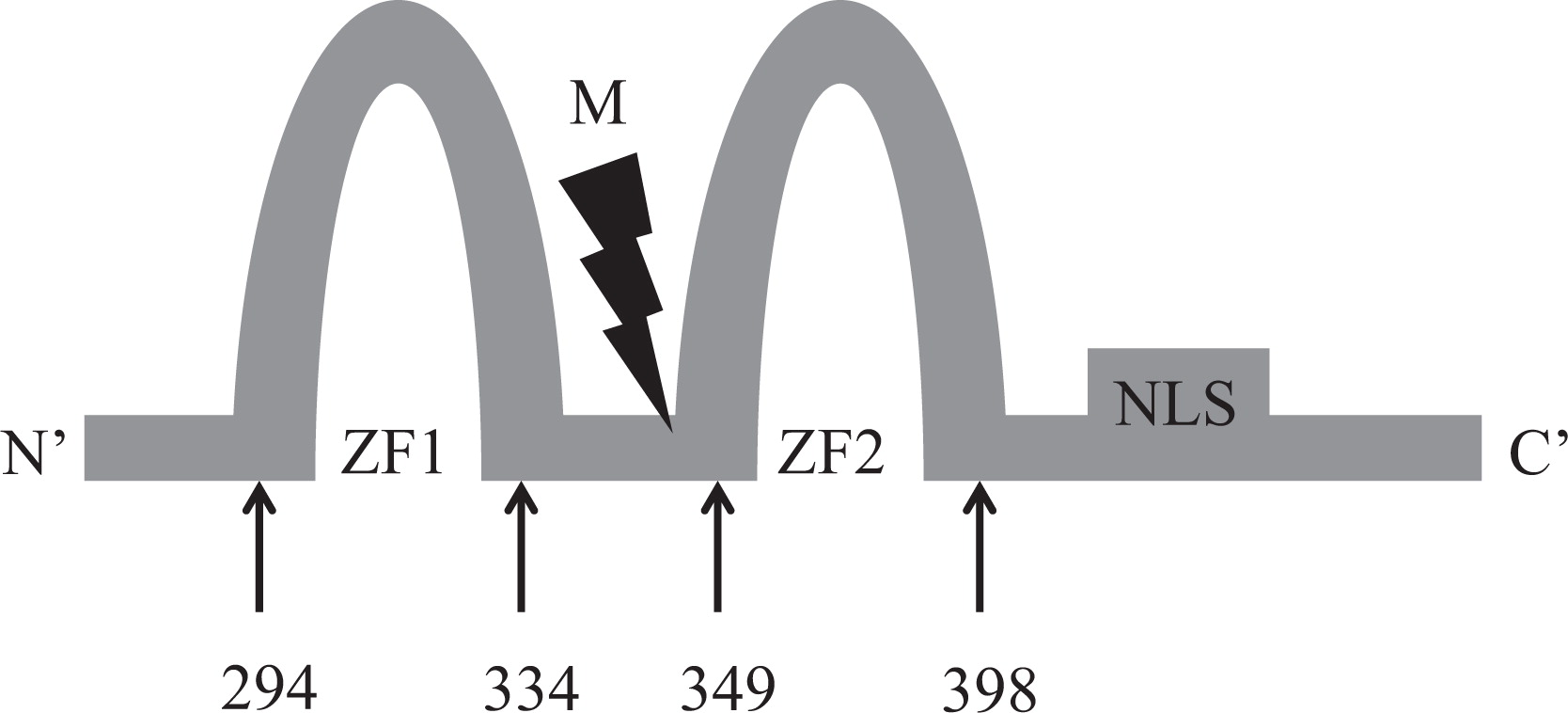
GATA2 gene, located at chromosome 3q21.3, encodes a dual zinc-finger transcription factor, known as GATA binding protein 2 that binds to the promoter and regulatory regions of target genes in the nucleus.
GATA2 regulates transcription of genes involved in the development and proliferation of diverse cell lineages, including hematopoietic, endothelial, placental, neuronal, etc. (
Bresnick et al. 2010). The impaired function of hematopoietic stem cells from
GATA2 +/− mice (
Rodrigues et al. 2005) and the association of hematological malignancies with translocation of
GATA2 enhancer elements (
Gröschel et al. 2014) suggest a dose-dependent effect for
GATA2 on hematopoiesis. Indeed, heterozygous
GATA2 mutations were recently identified among patients with diverse hematological and nonhematological manifestations. These conditions have been characterized as: (
i) monocytopenia and mycobacterial infections (MonoMAC) syndrome (
Hsu et al. 2011); (
ii) dendritic cell, monocyte, B and NK lymphoid (DCML) deficiency (
Dickinson et al. 2011); (
iii) familial myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) (
Hahn et al. 2011); and (
iv) primary lymphedema, congenital deafness, and MDS (Emberger syndrome) (
Ostergaard et al. 2011). Additionally,
GATA2 mutations predispose for pulmonary alveolar proteinosis, disseminated warts and human papillomavirus-associated squamous cell cancer (
Hsu et al. 2011), childhood neutropenia (
Pasquet et al. 2013), as well as NK-cell deficiency (
Mace et al. 2013). Recent analysis of 57 patients with
GATA2 mutations found a high correlation (
p < 0.001) between monocytopenia, B, NK, and CD4 lymphocytopenia and the presence of disease (
Spinner et al. 2014), emphasizing the importance of measuring monocyte numbers for the diagnosis of this condition. Indeed, 78.9% of this cohort had reduced monocyte numbers, 15.7% had normal monocyte numbers, and only 5.3% of the patients had increased monocyte numbers (
Spinner et al. 2014). However, this cohort as well as other reports of
GATA2-associated disorders included mostly teenagers and adult patients (
Pasquet et al. 2013;
Dickinson et al. 2014).
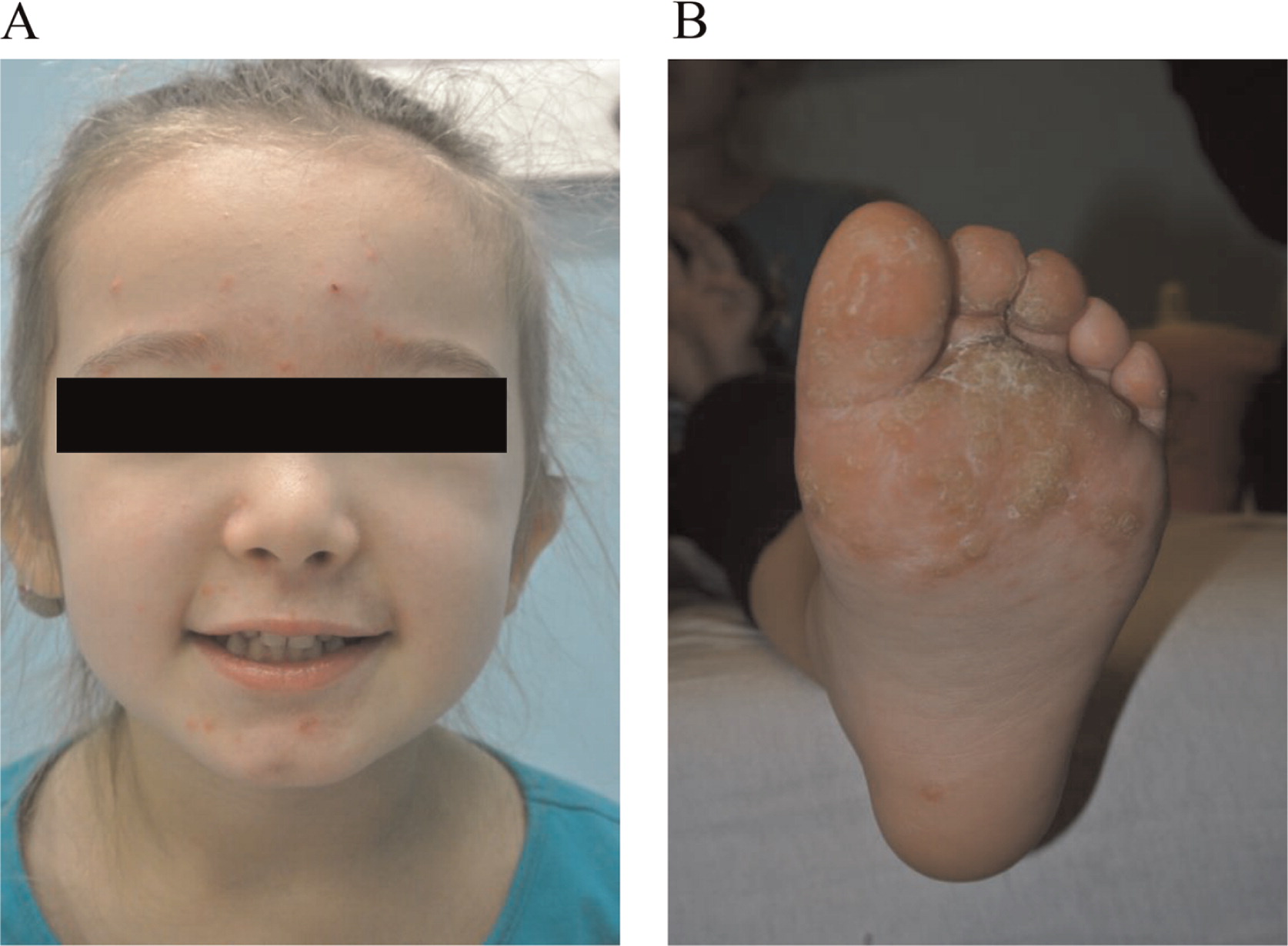
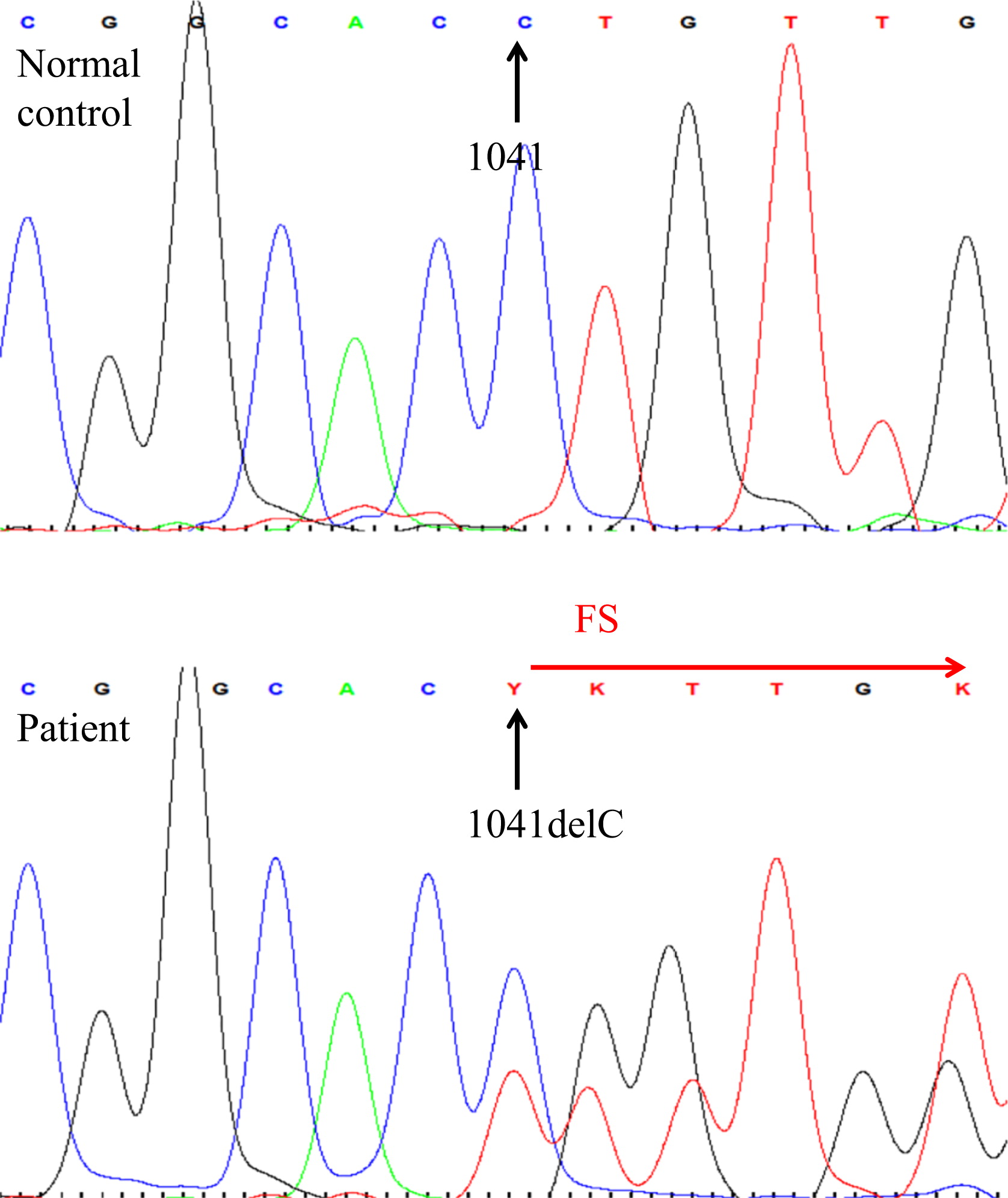
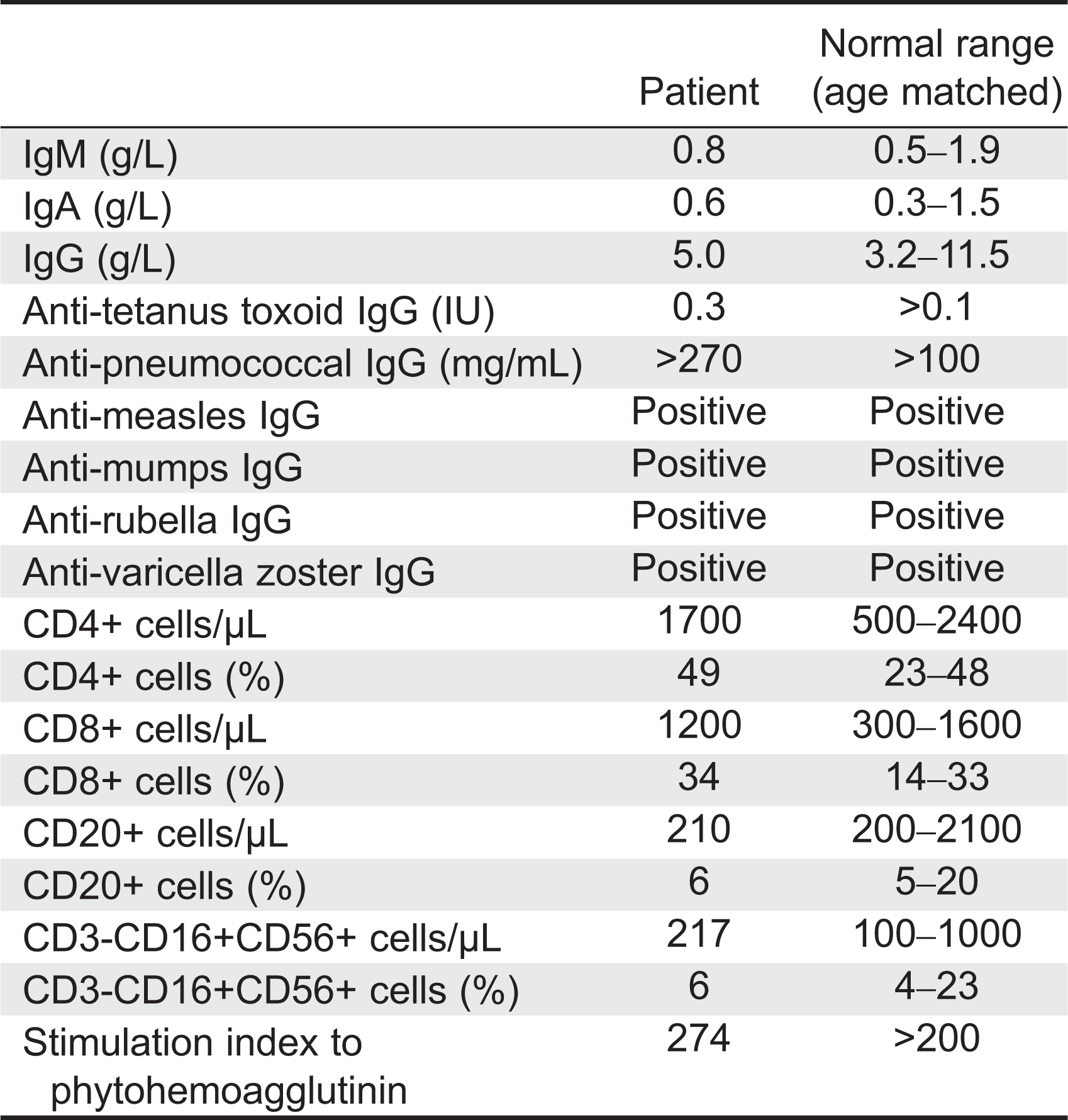
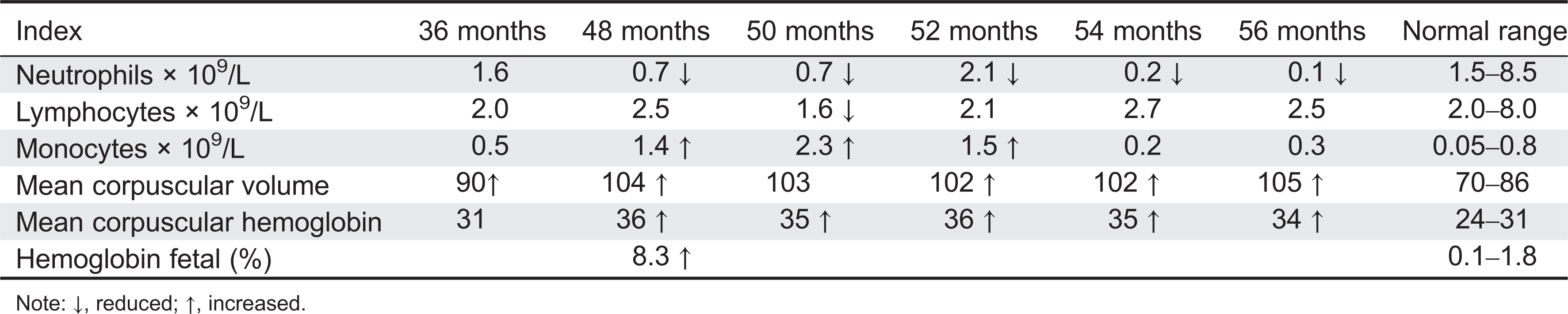
Here we report on a patient that presented in infancy with severe hearing loss and lymphedema. By 4 years of age the patient developed acne, disseminated warts, lymphadenopathy, and myelodysplastic syndrome. This patient had long-standing monocytosis with a novel GATA2 mutation.