Rationale (3.1)
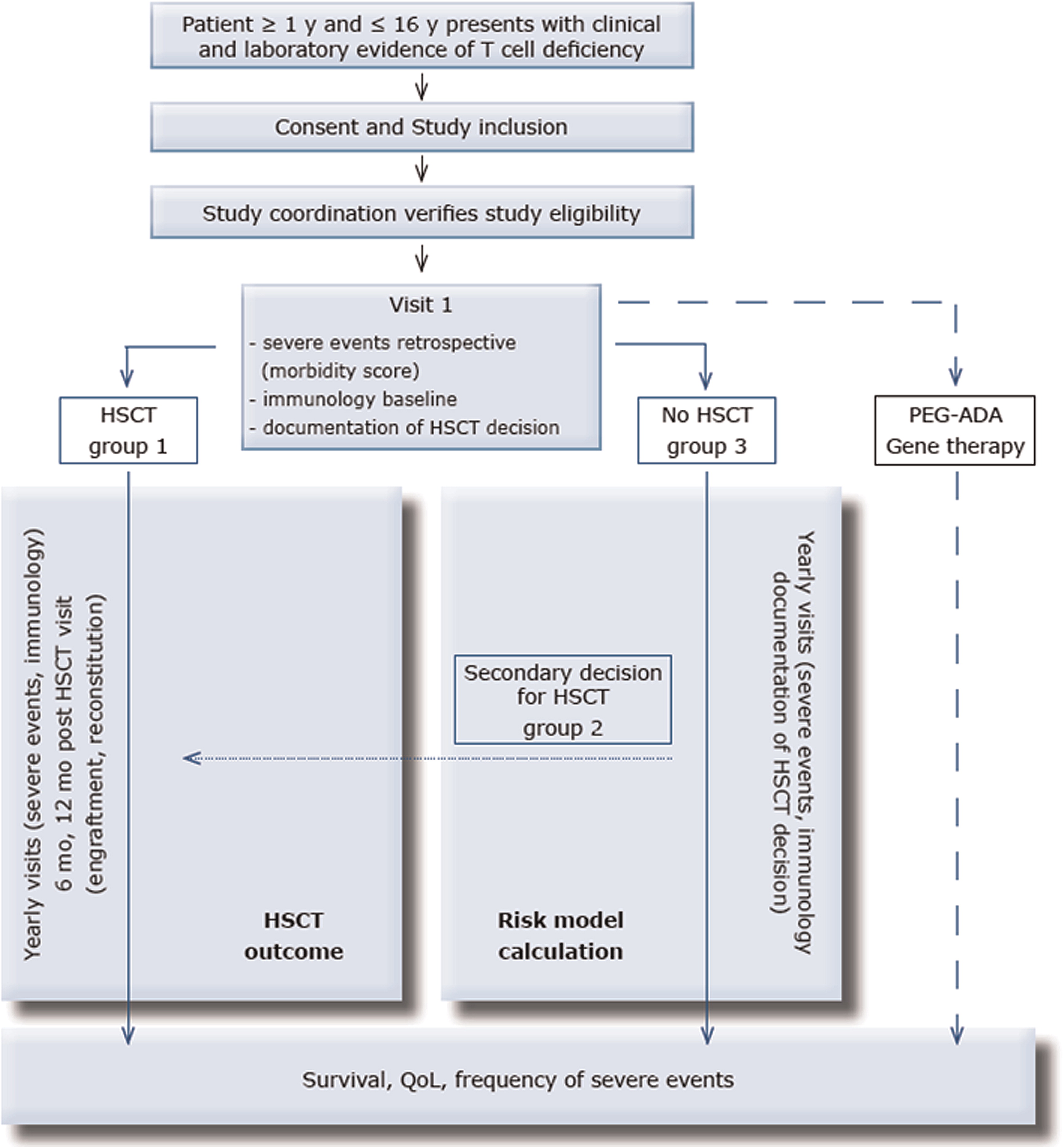
We postulate that the nature and the extent of the T-cell deficiency and their development over time are key factors determining the prognosis for CID patients that are to some extent independent of the particular molecular diagnosis. We argue that in addition to factors specific for certain molecular causes of CID such as radiosensitivity or syndromal manifestations, knowledge about the association of the degree of impairment of T-cell immunity with outcome (mortality, morbidity, quality of life (QOL)) will be helpful in making appropriate decisions on whether and when to transplant CID patients. We think that consideration of CID as a group of diseases is justified in this context, because many molecular diagnoses associated with CID are so rare that it is highly unlikely that treatment recommendations will be derived from larger cohort studies. In this study, we focus on children in whom HSCT is a relevant consideration at study entry, i.e., patients who have a documented T-cell deficiency that is profound enough to have caused a significant clinical manifestation such as a severe infection or a severe manifestation of impaired immune regulation. For the purpose of this study, these patients are classified as suffering from profound combined immunodeficiency (P-CID).
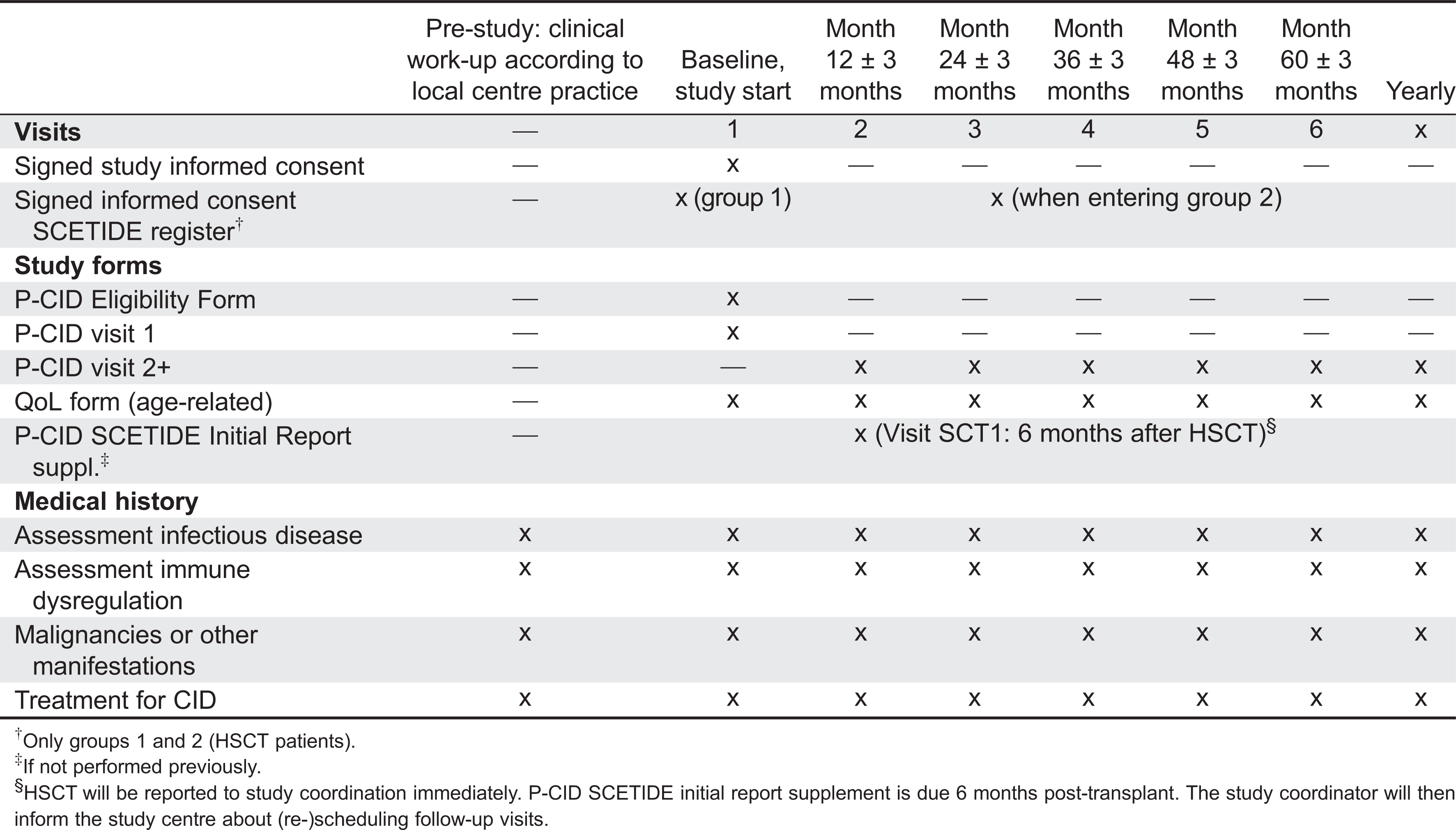
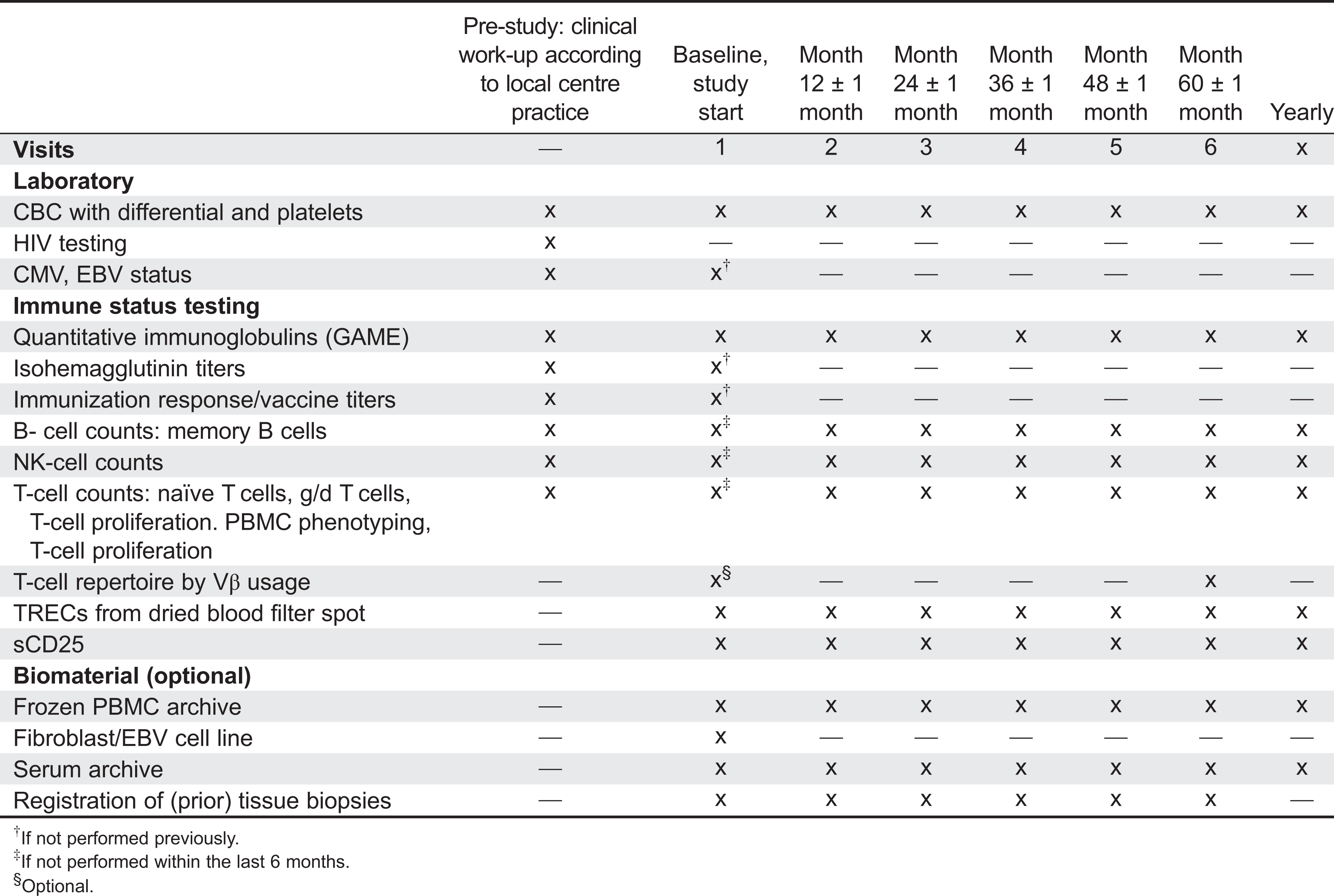
A prospective multicentre study of patients with P-CID has not been performed previously. The long-term outcome of patients with this condition therefore remains poorly characterized, and the appropriate treatment as well as the appropriate time point and criteria for interventions such as HSCT remain undetermined. This natural history study includes nontransplanted patients with P-CID who are treated according to centre policy by participating institutions. To determine the clinical and laboratory patient variables that are most important in determining outcome, we will uniformly collect clinical data and biomarkers on all patients entered into this study. At study entry, there will be a retrospective documentation of severe infections, severe immune dysregulation, and malignancies (severe events) and such events and survival will then be documented prospectively throughout the 5-year observation period.
Patients will be distributed among 3 strata: (i) patients for whom the decision to transplant is made at the time point of study entry (group 1, primary HSCT); (ii) patients who are initially followed without transplantation, but for whom a decision to transplant is made during the study period (group 2, secondary HSCT); and (iii) patients, who are not transplanted during the observation period (group 3, no HSCT). Patients receiving alternative therapies, in particular PEG-ADA or gene therapy, will be followed according to treatment-specific study protocols.
The American Primary Immune Deficiency Treatment Consortium (PIDTC) has recently conceived a Prospective Natural History Study of Diagnosis, Treatment, and Outcomes of Children with SCID Disorders (RDCRN PIDTC Protocol # 6901). That study concentrates mainly on patients with SCID (who are excluded in the P-CID study), but also includes patients with leaky SCID and Omenn syndrome. Although all patients in the U.S. study undergo HSCT at study entry, only a part of the patients in the P-CID study will be transplanted initially. Nevertheless, there is a significant patient group that would be eligible for the U.S. and the European study. It is our intention to collaborate with this U.S. initiative and to provide a data framework in the P-CID study that will allow comparison of the data obtained in the 2 studies. For this, all patients transplanted in the P-CID study will be entered into the stem cell transplantation for immunodeficiencies (SCETIDE) registry.
On the basis of this HSCT documentation, we aim to identify variables contributing to the best outcomes for HSCT, which is an important therapeutic option for patients with P-CID. Because P-CID includes a spectrum of immunologic presentations, and there have been several approaches to transplant, there are many questions of interest and variables to be explored. All patients undergoing primary or secondary HSCT will be included in this analysis.
The results of this study will help to make treatment decisions for patients with non-SCID T-cell deficiencies. In particular, it will provide information about the risks and benefits of HSCT in P-CID patients and about the time point when HSCT is indicated. In collaboration and extension of the U.S. study, our investigations will also provide data that defines optimal conditions for the achievement of immune reconstitution. The results will also shape future directions for trials of HSCT and other cellular therapies applied to P-CID patients.