Introduction
X-linked agammaglobulinemia (XLA), or Bruton's disease (
Bruton 1952), is a rare immunodeficiency in which mutations in the
BTK gene cause an arrest of B-cell development with a lack of peripheral mature B cells and profound hypogammaglobulinemia. As a result, patients have an increased susceptibility to a variety of encapsulated bacteria and enteroviruses; microorganisms for which antibodies play a critical role in host defense. According to a U.S. registry, the most common presentations leading to diagnosis were: infections in 85% of patients (most common), positive family history (second most common), and neutropenia (third most common) (
Winkelstein et al. 2006). The most common infections are sinopulmonary: 50% of the patients present by 1 year of age and almost all of the patients present by the age of 5 years. The most common pathogens are
Streptococcus pneumoniae,
Haemophilus influenzae, and
Pseudomonas spp. However, some patients have an atypical presentation. Because XLA is a rare disease, relatively few studies reporting atypical presentations have been published, most of them show late presentation, and very few describe atypical presentation in the young age group (
Hashimoto et al. 1999;
Stewart et al. 2001). Among these unusual presentations are reports of patients with pyoderma skin lesions (
Lin et al. 2006), and
Pneumocystis carinii pneumonitis (
Saulsbury et al. 1979). Studies attempting to investigate genotype–phenotype correlation suggested that missense mutations in the
BTK gene that affect nonconserved regions of the molecule are less severe (
López-Granados et al. 2005) and that mutations that preserve expression of the protein usually have a higher number of B cells (
Broides et al. 2006); however, there are many exceptions to these rules.
In this article we present 4 patients with unusual presentations or manifestations, some of which are unexpected in XLA patients such as autoimmune phenomenon.
Results
Patient 1
This patient was born after a normal pregnancy to nonconsanguineous parents and presented at 15 months of age with fever, rash, and neutropenia. Five months later he experienced another febrile illness and again blood film showed he had neutropenia. On examination he had no dysmorphic features, weight and height were at the 75th percentile, and development was appropriate for his age. He later developed recurrent pruritic erythematous papular lesions on his trunk and extremities. Biopsies revealed multiple granulomas with scattered Langerhans-type giant cells and extensive mononuclear infiltration consistent with granulomatous disease.
Bacterial, mycobacteria, and fungal stains were negative. The patient did not have a fever, and he had no increased inflammatory markers. Thorough radiologic evaluation did not reveal evidence for visceral granulomatous disease. The skin lesions slowly resolved with topical steroids and anti-bacterial treatment.
Patient 2
This patient was a 9-year-old boy who was transferred to our hospital with intractable seizures. His illness followed an upper respiratory viral disease. A week after his acute febrile illness, which was considered an upper respiratory infection, he developed ataxia, dysarthria, and dysphagia without fever. The initial sepsis workup revealed a white blood cell count of 2.6 × 103 cell/µL, and lumbar puncture showed cerebral spinal fluid (CSF) with a high protein level of 889 mg/L (100–400 normal range) but no elevation of white blood cell count, normal glucose, and no organisms in the gram stain or cultures (before initiation of any antimicrobial treatment). Imaging of his brain showed areas of demyelination that were typical of acute demyelinating encephalomyelitis.
Past medical history revealed one occasion of suspected pneumonia that was treated with a course of erythromycin, and it resolved quickly. Physical examination revealed small cervical lymph nodes, and tonsillar tissue was present.
Family history was unremarkable with nonconsanguineous parents and 3 healthy siblings. There was no history of recurrent infections or early childhood death in the family.
The patient was treated with steroids, acyclovir, dilantin, and intravenous immunoglobulins (IVIg); he slowly and fully recovered and continues on IVIg treatment.
Patient 3
This patient was a boy, 3 years and 10 months of age, who was diagnosed initially because of a recurrent, red, fine, nonitchy rash that persisted for a week involving the trunk and extremities including palms and soles. During early events he was afebrile and well, but later on these episodes were associated with fever. Serology tests for viruses, including measles, which the child was vaccinated for, were negative. During the period of investigation he developed thrombocytopenia (platelets 40 000 cells/µL) and a bone marrow aspiration showed normal cellularity with adequate numbers of megakaryocytes as well as normal erythropoiesis and granulopoiesis. Upon starting replacement with IVIg, the thrombocytopenia resolved suggesting an underlying autoimmune mechanism.
The child's past medical history revealed several episodes of acute otitis media that resolved after the insertion of tympanic ventilation tubes. He did not experience pneumonia or other invasive infections. He did not require intravenous antibiotics treatment at any time.
Family history did not reveal any individuals with recurrent infection or death in early childhood caused by infections. On physical examination, small cervical lymph nodes were palpated and tonsillar tissue could be clearly visualized.
Patient 4
This patient was a boy who presented at the age of 1 year 10 months with diarrhea lasting for 2 months. An initial investigation recovered Giardia Lamblia from the stool and treatment with Flagyl resulted in improvement. Six months later he developed fever, perianal ulcers, and bloody stools. No infectious cause was found and gastrointestinal (GI) studies revealed thickening of the bowel wall and numerous pseudopolyps.
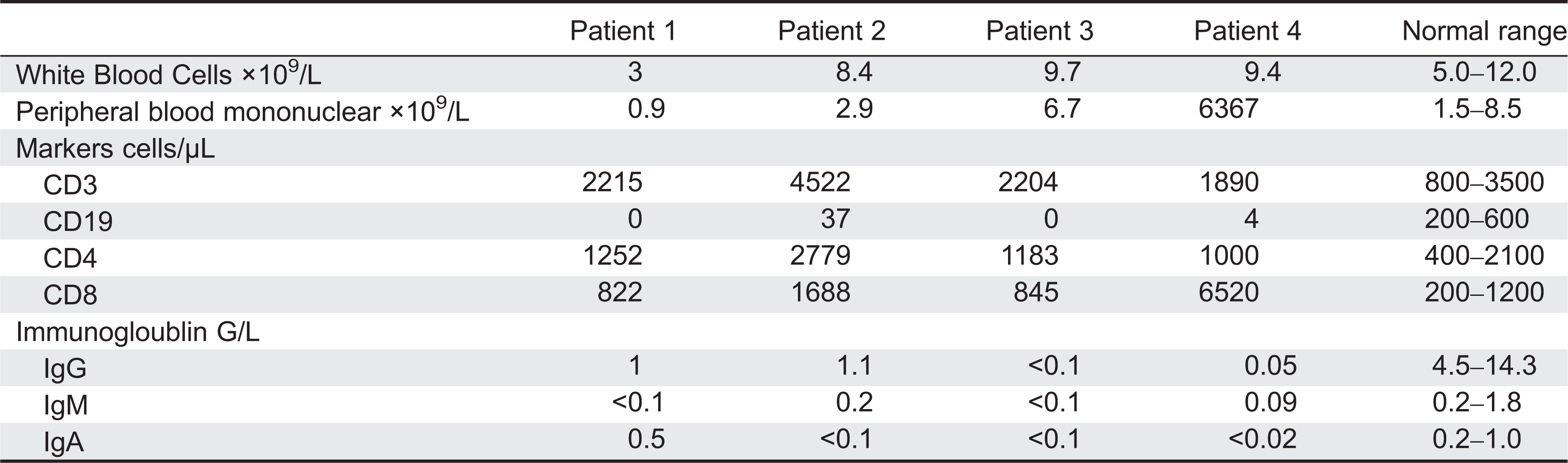
Immune workup at this point revealed that immunoglobulins were absent. Flow cytometry showed near-absence of B lymphocytes, 0.2% of the total number.
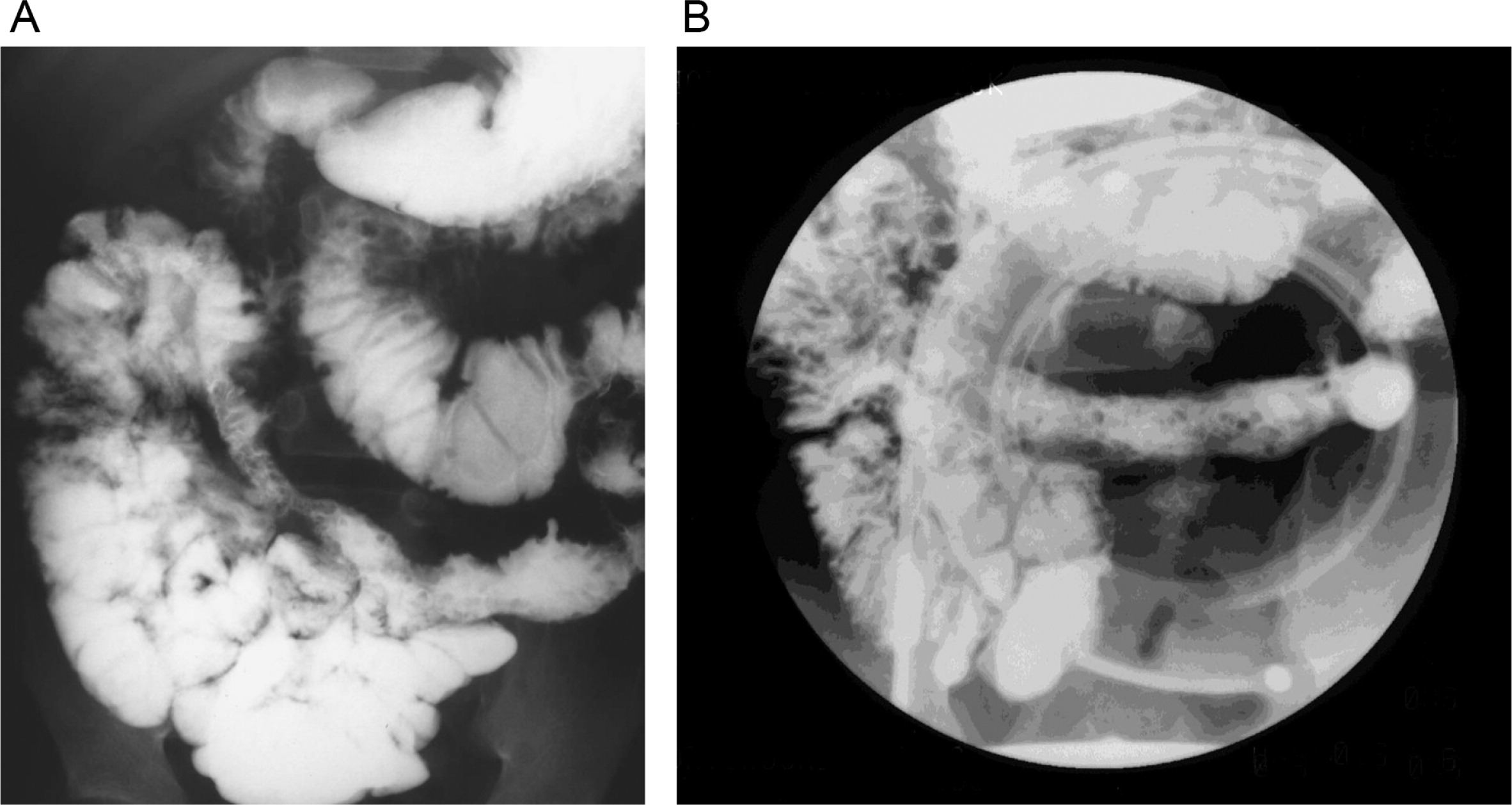
He continued to experience bouts of diarrhea, leading to failure to thrive. Further studies showed protein-losing enteropathy and repeated endoscopies with biopsies showed distortion of villous architecture with moderate crypt hyperplasia, absence of plasma cells, and decreased lymphoid cells in the lamina propria. At the age of 13 years he was admitted for left-sided abdominal pain and was noted to have lower left abdominal tenderness. Bowel contrast studies (
Figures 1–
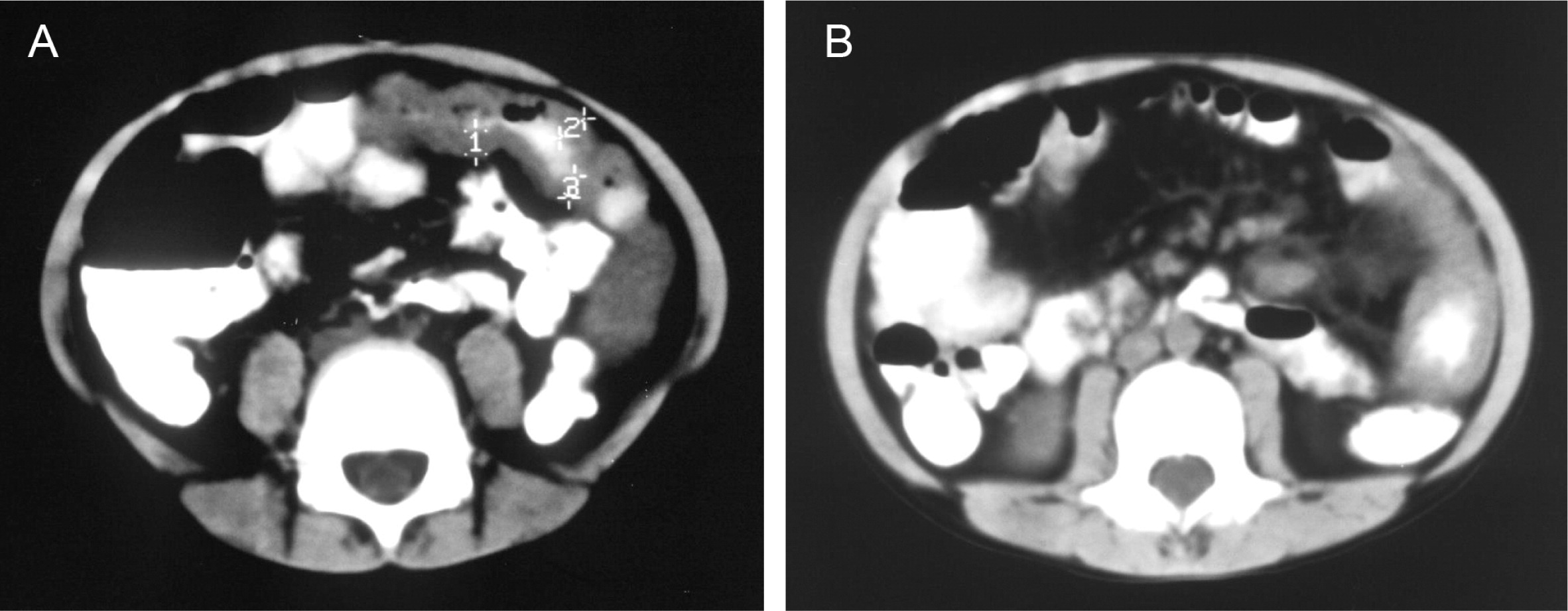
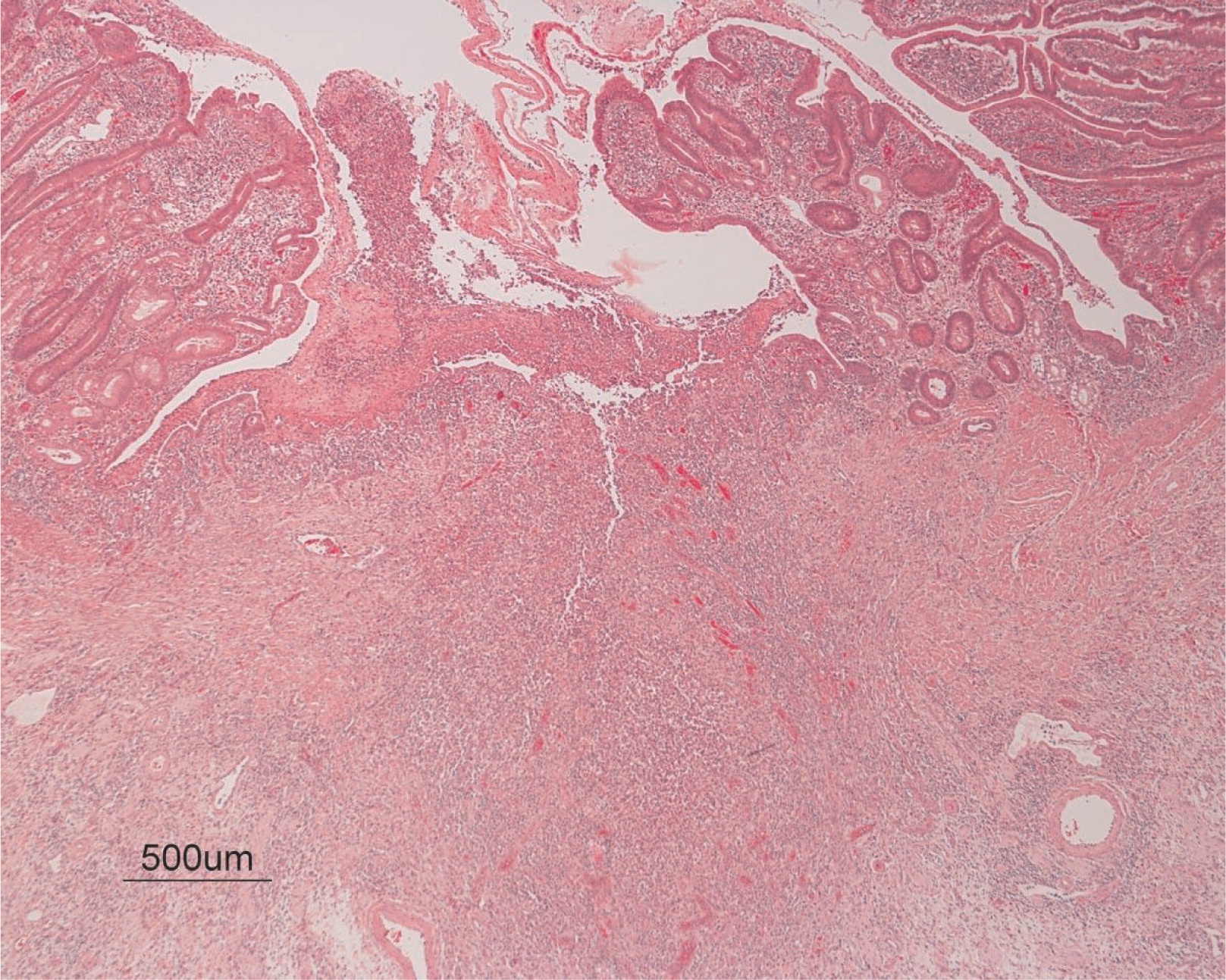
3) revealed a consistent filling defect of mid-jejunum and a CT scan demonstrated focal small bowel thickening and a possible submucosal process, dubbed “Crohn's like”. Symptoms intensified and he underwent a laparotomy during which a 77 cm segment of jejunum was resected. Examination of the resected segment demonstrated numerous pseudopolyps with extensive foci of ulcerations, congestions, and hemorrhages, with total effacement of mucosal architecture (
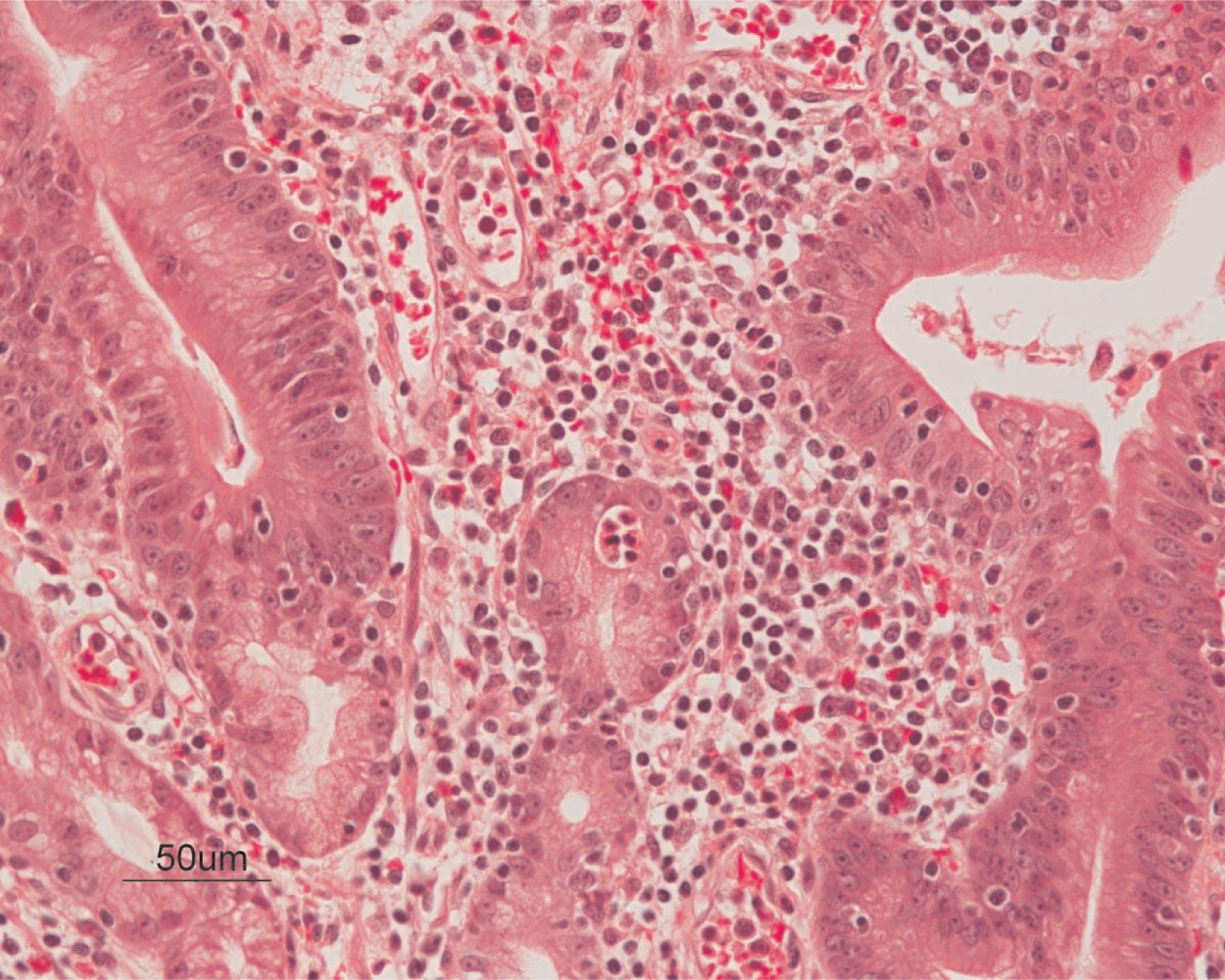
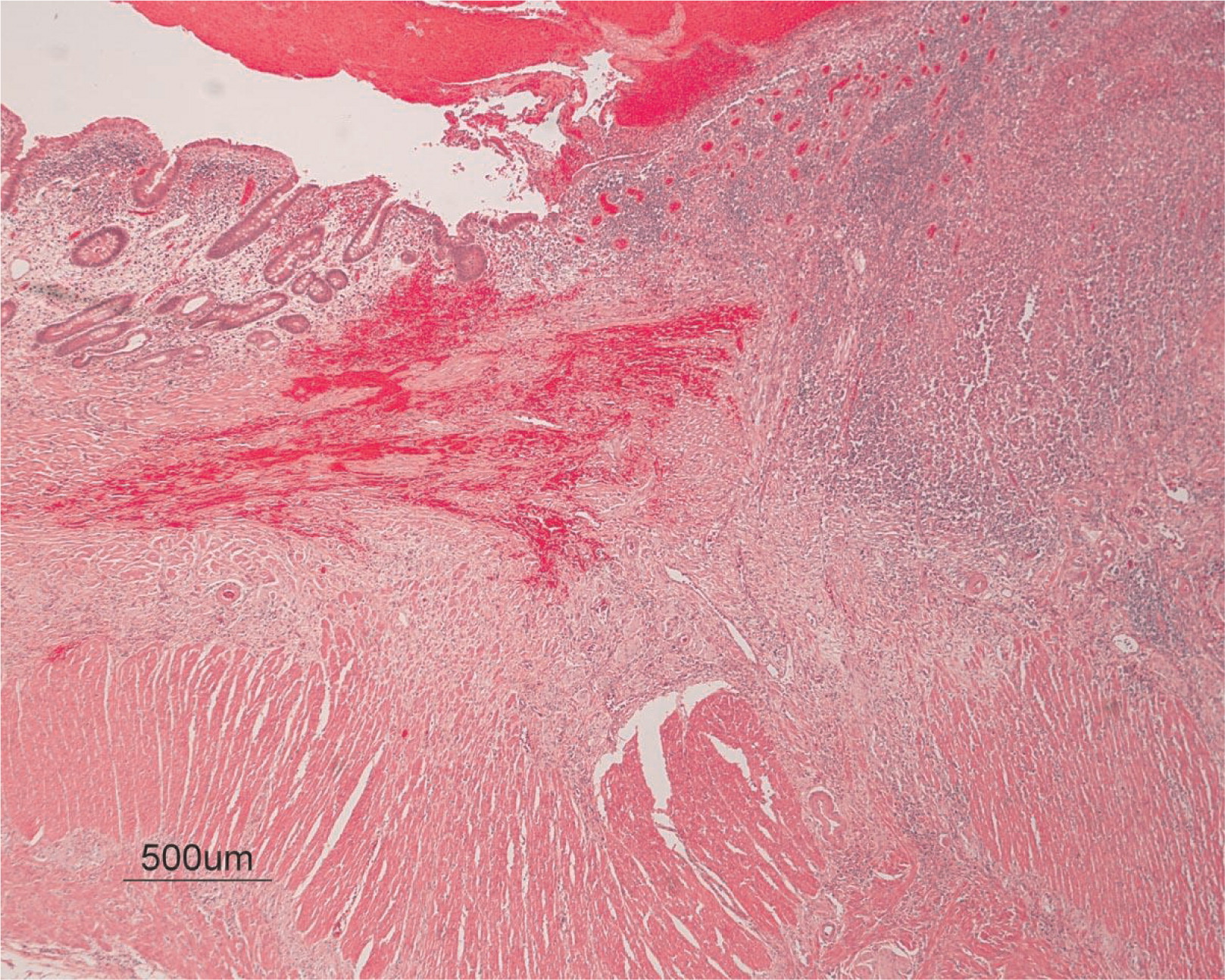
Figure 4). On microscopy, the ulcerated segment revealed acute on chronic inflammation. The acute inflammation was characterized by the presence of cryptitis (
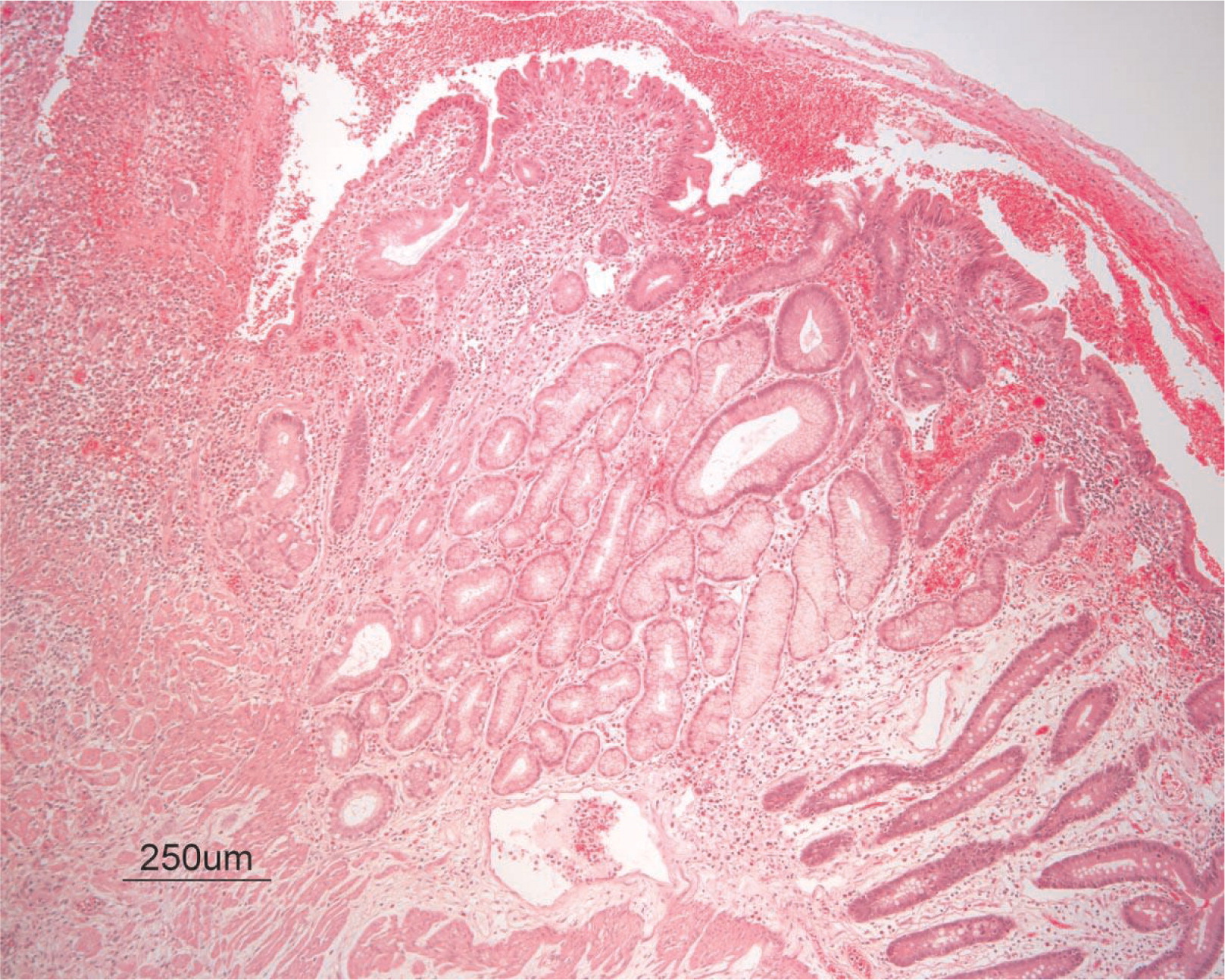
Figure 5). The lamina propria was expanded by increased numbers of mononuclear cells but no plasma cells were identified. In addition to the presence of increased infiltrates of chronic inflammatory lymphocytes, the lamina propria also showed underlying chronic regenerative changes such as glandular branching, focal reduplication of the muscularis mucosae, and hypertrophy of the muscularis propria (
Figures 6 and
7). No granulomas or granulomatous inflammatory infiltrates were found. In addition, gastric metaplasia was seen in the submucosa (
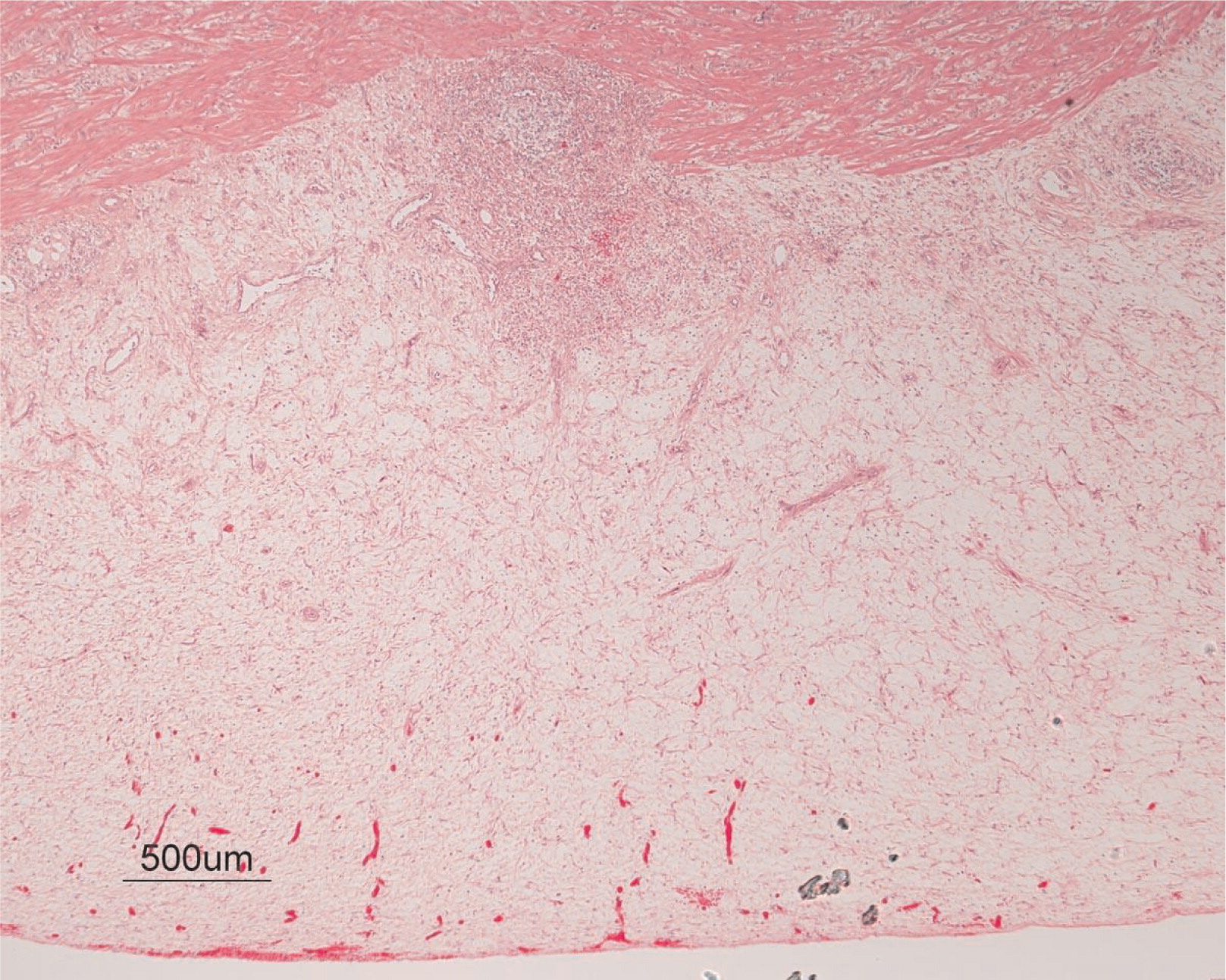
Figure 7). In some areas of the ulcer the muscosa was replaced by acutely inflamed granulation tissue (
Figures 4 and
7). Intense infiltrates of neutrophils extended from the ulcerated lamina propria deep into the submucosa and through the muscularis propria (
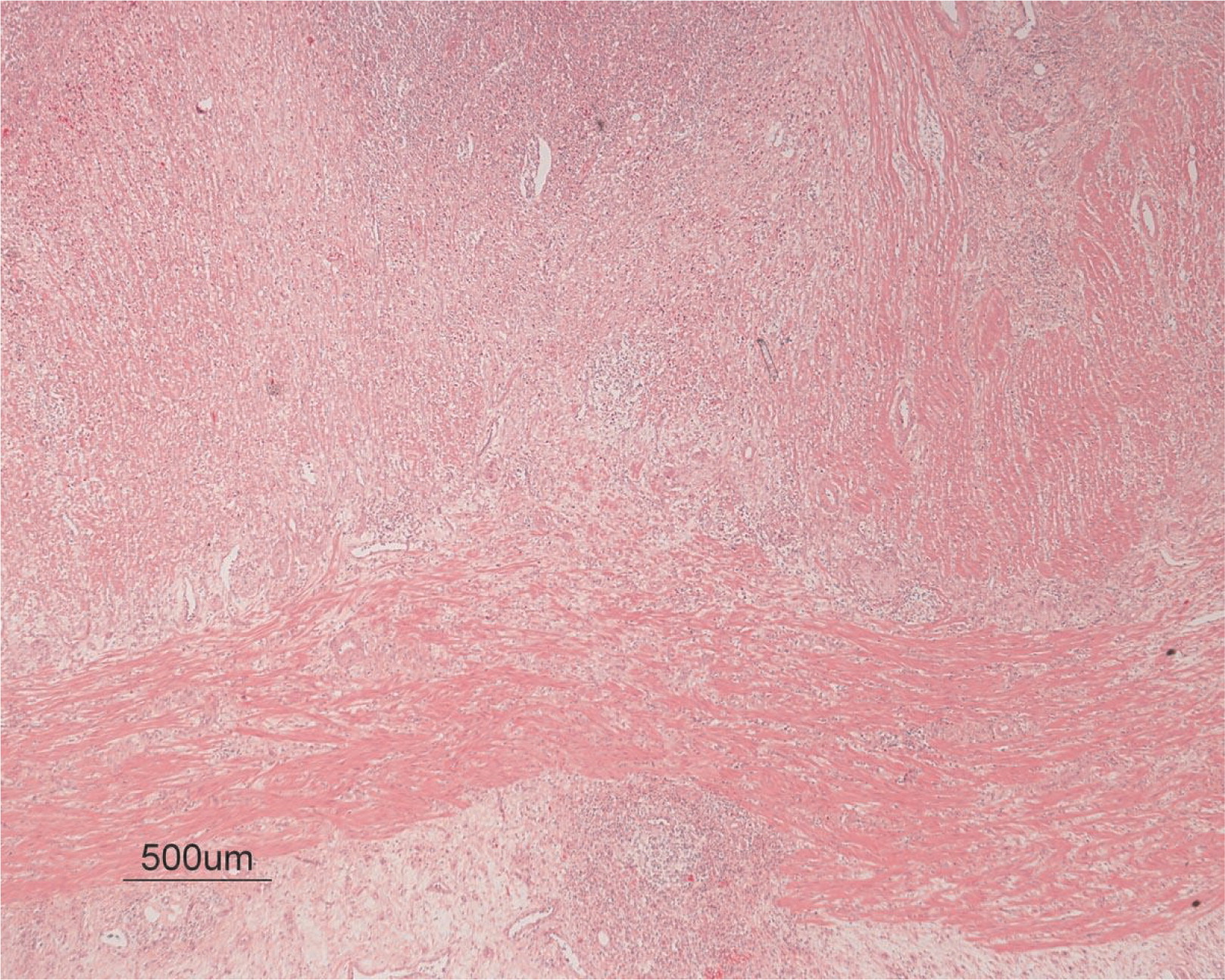
Figures 7 and
8). The inflammation resulted in the development of edema, mild fibrosis, and thickening of the serosa (
Figure 9). All of these changes in the bowel wall correlated with the Crohn's-like radiologic changes that were detected. Despite the pan-mural ileitis, a diagnosis of Crohn's ileitis could not be made because of the absence of granuloma formations. The diagnosis of bacterial overgrowth was also not supported as all bacterial, fungal, and protozoal stains were negative.
Following the resection, a complete resolution of the symptoms including protein-losing enteropathy ensued. Ten years later he remained completely symptom-free from GI complaints.
Genetic analysis
Despite the unusual clinical presentation, the low number of circulating B cells as well as the low levels of immunoglobulin prompted the sequence analysis of the
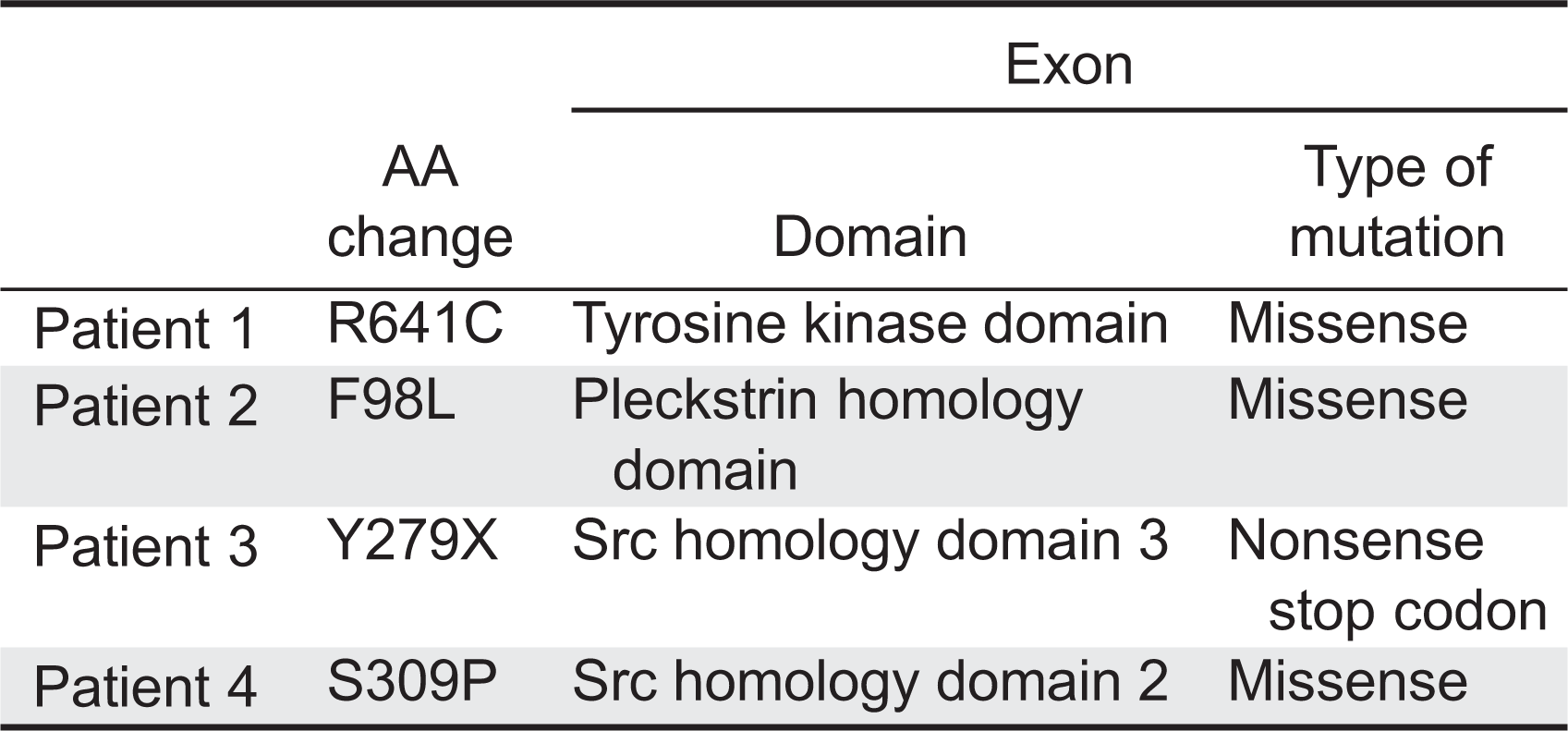
BTK gene in these patients. One patient had nonsense mutation, whereas 3 other patients had missense mutations (
Table 2).
Patient 1 had a C to T transition at position 1921, which predicts an arginine to cysteine substitution at position 641 in the kinase domain.
Patient 2 had a T to C exchange at position 98, which predicts a phenylalanine to leucine substitution.
Patient 3 had a T to G transition at position 279, creating a stop codon in the SH3 domain of BTK.
Patient 4 had a T to C transition at position 925 resulting in a serine to proline exchange at position 309 in the SH2 domain.
Discussion
Typically, XLA presents in infancy or childhood with repeated microbial infections and a complete lack of tonsils and lymph nodes. However, several cases with atypical presentation in adult life have been reported. In our cohort of patients with XLA we have identified 4 patients with unusual and unique presentations or manifestations. Invasive infections were uncommon, responded well to antibiotic treatment, or resolved after myringotomy tube insertion. Such features are common in the pediatric population and therefore did not trigger an assessment for primary immunodeficiency. In addition, small cervical lymph nodes were present in some cases, which may have misled the primary care physician and ear, nose, and throat specialist.
The main presentation from patient 1 was unusual granulomatous skin eruptions. Granulomatous Dermatitis (GD) is defined by an infiltration of mononuclear and multinucleate giant cells in the skin, with or without lymphocyte infiltration (
Mohan et al. 2006). There are several histopathological types of granulomatous reactions in the skin and they include tuberculoid, sarcoid, necrobiotic, suppurative, foreign bodies, and other rare causes such as primary or secondary immunodeficiencies. In infants and young children GD may be associated with infectious caused by mycobacteria, bacteria, fungi, or viral agents (
Goodless et al. 1991;
Gibney et al. 1996;
Eisendle and Zelger 2009), and it may be linked to various medications such as calcium channel blockers, ACE inhibitors, and others (
Magro et al. 1998). In older children and adolescents, it can be associated with autoimmune diseases such as sarcoidosis, rheumatoid arthritis, systemic lupus erythematous (
Rose et al. 2014), or inflammatory bowel disease. GD has also been described in immunodeficiency states such as chronic granulomatous disease and common variable immunodeficiency (CVID) (
Torrelo et al. 1995). None of these associations could be identified in our patients. It therefore remains unclear why this patient was afflicted with GD. It is possible that this aberrant inflammatory process might be related to effects stemming from
BTK dysfunction in a variety of cell types such as monocytes and dendritic cells (
Koprulu and Ellmeier 2009). XLA patients have a high percentage of CD14
+CD16
+ and CD14 CD35
+ cells, but phagocytosis and chemotaxis by these cells seems reduced (
Amoras et al. 2007). Moreover, monocytes from
BTK-deficient patients showed an increased secretion of interleukin (IL)-6, IL-10, and tumor necrosis factor (TNF)-α in response to TLR 4, 7, and 8 stimulation (
Marron et al. 2012), whereas cytokine secretion upon stimulation of dendritic cells following stimulation of TLR9 was significantly reduced (
Lougaris et al. 2014). All these abnormalities that resulted in distorted inflammatory responses may have contributed to the formation of these granulomas in the patients with XLA.
Acute demyelinating encephalomyelitis (ADEM) is considered an infection related autoimmune demyelinating disease of the central nervous system. Pathogenesis of the disease is probably autoimmune and there are several proposed mechanisms. One leading theory is based on the assumption of molecular mimicry, involved structural, or partial amino-acid sequence homologies, which is shared between a pathogen and a host central nervous system (CNS) protein. In such a scenario, initial immune response to the pathogen leads to T-cell activation, which in turn cross-activates antigen-specific B cells creating auto-reactive T and B cells. These cells are capable of entering the CNS) and may recognize a homologous myelin protein (even long after clearance of the pathogen) eliciting an inflammatory reaction.
Another explanation might be a post-infectious etiology, whereby a direct CNS infection causes tissue damage and a disruption of the blood–brain barrier. This may lead to leakage of CNS-privileged auto antigens that could be processed in systemic lymphatic organs, eliciting a self-reactive encephalopathic T-cell response. In patient 2 a viral illness may have led to development of this severe sequel. Although viral meningoencephalitis due to ECHO and other viruses is a common finding in XLA patients, the infection-related or autoimmune encephalitis such as ADEM is uncommon. This may have been due to an aberrant immune reaction owing to
BTK deficiency. Although T cells are responsible for most antiviral responses, B cells and antibodies may be critical in the response to some viruses such as enteroviruses, hence the increased susceptibility of XLA to echovirus (
Halliday et al. 2003). However, viruses were not detected in the CSF obtained from this patient. Nevertheless this possibility cannot be completely excluded.
Another unusual manifestation for XLA was idiopathic thrombocytopenic purpura (ITP), identified in patient 3. This is a common putatively autoimmune phenomenon in other immune deficiency states such as CVID and autoimmune lymphoproliferative syndrome but not in XLA. Using the common assumption that ITP is caused by auto antibodies, it was a surprising occurrence in a patient who has difficulties producing any antibodies. This may suggest some residual ability in XLA patients to produce antibodies. In fact, a short time after starting replacement with IVIg the thrombocytopenia resolved, thus supporting the possibility of an underlying autoimmune mechanism.
Finally, patient 4 had a prolonged course of Crohn's-like inflammatory bowel disease or infantile colitis with a prominent feature of protein-losing enteropathy. The bowel lesion was limited to a jejunal segment that upon resection led to complete resolution of all symptoms. Although the findings from the biopsies were showing inflammation, they were not of the granulomatous type and were thus incompatible with classic Crohn's disease. Chronic noninfectious inflammatory bowel disease manifesting as small intestine irregularities and strictures have been previously described in a few patients with XLA (
Abramowsky and Sorensen 1988;
Washington et al. 1996). With the recent discovery of several monogenic disorders such as FOXP3, IL-10, and IL-10R leading to infantile colitis, it is important to remember that XLA may present in a similar manner during infancy.
In this group of patients we saw manifestations that are atypical to XLA, in particular autoimmune phenomenon that are not known to be common in XLA patients.
Previous studies (
López-Granados et al. 2005;
Broides et al. 2006) suggested that a milder presentation may be related to missense mutations. In our group of patients we observed mutations in all regions of the gene with both missense and nonsense mutations. Despite having a nonsense mutation, patient 3 had a relatively mild course including a rash and later ITP. The small number of patients in this group precludes accurate statistical analysis, but it supports the previous notion of no clear genotype–phenotype correlation in XLA patients.
In summary, these 4 patients with novel mutations had a variety of unique inflammatory and autoimmune clinical manifestations that should alert physicians and immunologists to the possibility of XLA in similar cases.