Introduction
Adenosine deaminase (ADA) is a ubiquitously expressed enzyme of the purine salvage pathway which catalyzes the irreversible deamination of adenosine and deoxyadenosine. It is essential for normal lymphoid development, with particularly high levels found in the thymus (
Adams and Harkness 1976;
Poliani et al. 2009). Deficiency of ADA, caused by mutations in the
ADA gene and subsequent impairment of ADA activity, leads to an autosomal recessive form of severe combined immunodeficiency (SCID) (
Sauer et al. 2012;
Bradford et al. 2017). The toxic accumulation of ADA substrates and metabolites interferes with downstream metabolic pathways, including inhibition of ribonucleotide reductase and subsequent blockade of DNA synthesis, as well as S-adenosylhomocysteine hydrolase-dependent transmethylation (
Flinn and Gennery 2018). The detrimental effects are most pronounced in pathways regulating lymphocyte maturation and function, although non-immunological organ systems, including the hepatic, renal, pulmonary, skeletal, peripheral and central nervous systems, can also be affected (reviewed by(
Whitmore and Gaspar (2016)).
ADA deficiency affects 1:200 000 live births (
Blackburn and Kellems 2005), with a higher frequency reported in Canadian Inuit and Mennonite populations (
Grunebaum et al. 2013). Without treatment it is usually fatal within the first year of life. Genotype-phenotype correlations have revealed greater metabolic disturbance in those with more severe biallelic defects in
ADA (
Arredondo-Vega et al. 1998;
Cagdas et al. 2018). By contrast, hypomorphic mutations result in somewhat reduced ADA activity and are associated with less severe or late-onset phenotypes. Prior to the advent of newborn screening, patients with complete ADA deficiency traditionally presented during infancy with recurrent bacterial, viral and fungal infections and failure to thrive, while laboratory evaluations revealed severe lymphopenia, hypogammaglobulinemia, and neutropenia. Skeletal abnormalities, cognitive impairment, and hearing loss are common (
Albuquerque and Gaspar 2004;
Titman et al. 2008;
Sauer et al. 2009;
Manson et al. 2013). Those with incomplete (partial) deficiency may present with milder but gradually worsening manifestations, associated with reduced populations of T, B, and NK cells (
Santisteban et al. 1993;
Shovlin et al. 1993). In either case, measurement of ADA enzyme activity and associated levels of ADA substrates/metabolites (adenosine, 2′deoxyadenosine (dAXP), deoxyadenosine triphosphate) is often the first step in identifying ADA deficiency.
The implementation of newborn screening for SCID in Canada (first introduced in Ontario in 2013 and now routine across Nova Scotia, New Brunswick, Prince Edward Island, and Alberta (
Biggs et al. 2017;
Reid et al. 2017)) has resulted in the early detection of ADA deficient patients as well as improved survival of this cohort (
Scott et al. 2019). Management currently relies on immediate enzyme replacement therapy (ERT), immunoglobulin replacement, protective isolation procedures, and antibiotic prophylaxis until definitive treatment is initiated (
Figure 1). Patients require close monitoring to reduce the risk of infectious and non-infectious complications (
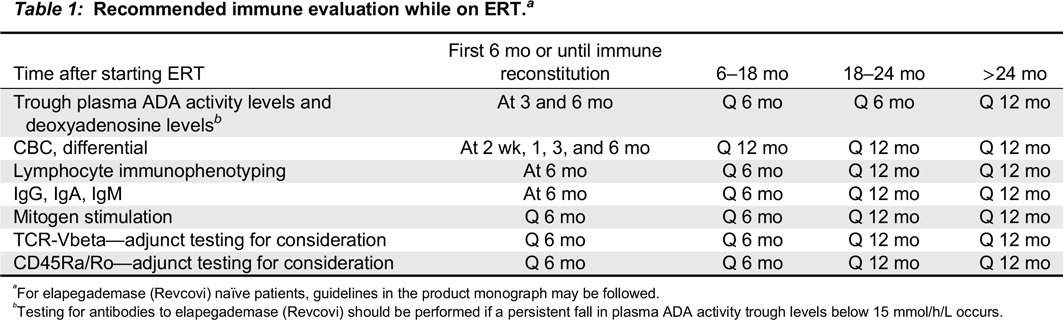
Table 1).
ERT using polyethylene glycol-modified bovine ADA has proved to be effective in correcting the immune and some non-immune abnormalities conferred by ADA deficiency, however, in some patients immune function declined over time (
Booth et al. 2007). The bovine-derived ADA product was replaced in 2019 by a recombinant form of the enzyme (elapegademase) which has shown similar in-vitro and possibly better in-vivo activity compared to its predecessor (
Murguia-Favela et al. 2020). ERT remains a costly treatment. Thus, it is considered a bridging modality before curative treatment is initiated. To date, hematopoietic stem cell transplantation (HSCT) is the treatment of choice when a human leukocyte antigen (HLA)-matched related donor (MRD) is available (
Griffith et al. 2008). Another option that has been shown to correct for the absence of ADA is ex vivo-modified autologous hematopoietic stem cell gene therapy (HSC-GT) (
Kohn et al. 1995;
Aiuti et al. 2002;
Ferrua et al. 2010;
Cicalese et al. 2016). Experiences with HSCT with matched unrelated donors (MUD) or haploidentical donors have shown lower success rates (
Hassan et al. 2012), although these options can still be considered if MRD HSCT or HSC-GT are not available.
Herein we, the Canadian Expert Committee, define guidelines on the management of ADA deficiency based on our collective experience and available literature in managing this group of patients.