Introduction
Mitogens, such as phytohemagglutinin (PHA), are plant lectins that are potent inducers of T cell proliferation (
Nowell 1960). These non-specific stimuli activate the surface glycoprotein of T cell receptors and initiate intracellular signaling, ultimately triggering cell division (
Sharon and Lis 2004).
For decades, assessment of lymphocyte proliferation has been a useful tool in the diagnosis of severe combined immunodeficiency (SCID). Patients with SCID invariably have low to absent responses to PHA (
Roifman et al. 2012). However, in patients with combined immunodeficiency (CID), the results of these tests have not been standardized and are open to a wide range of interpretations (
Roifman et al. 2012). Moreover, despite its use for over 50 years, there are currently no established reference ranges and the interpretation of test results is based upon comparison to healthy controls. This introduces multiple limitations, including variability associated with individual control samples, challenges of recruiting volunteers, and the additional labour associated with assaying control samples.
Given that the number of newly discovered CIDs continues to expand, improved interpretation of lymphocyte proliferation responses to mitogens, such as PHA, would aid in identifying such disorders.
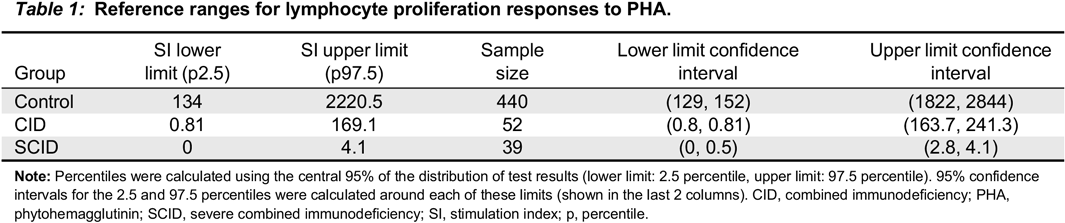
In this study, we collected retrospective data of lymphocyte proliferation responses to PHA from 39 patients with SCID, 52 patients with CID, as well as 440 healthy controls. Our primary goal was to establish reference ranges for patients with T cell defects, especially in those with CID. Importantly, identification of the gene defect associated with these T cell disorders has allowed, for the first time, critical analysis of mitogen responses in the corresponding conditions. We have subsequently studied in a prospective manner the validity of these reference ranges in patients with SCID or CID.
Discussion
Different centres have adopted varied methods for interpreting results of lymphocyte proliferation responses. Some have used an arbitrary threshold of counts from 3H-thymidine incorporation to distinguish normal from abnormal response, with most setting up side-by-side patient and normal control samples to be used as comparators. Regardless of the method used, they were all accurate in detecting typical SCID samples which were almost invariably extremely low. However, with the expanding recognition of a wide spectrum of T cell immunodeficiencies (CID), this type of analysis has posed increasing challenges in defining abnormal responses to mitogens.
One solution adopted by many centres was to arbitrarily define a response as normal if the patients’ lymphocyte stimulation index exceeded 50% of control. We found this method often times inadequate because of the great variability in single samples obtained from control and normal individuals.
In our study, SI of healthy controls ranged from 134 to 2220.5, demonstrating the wide range of the results of this test. Importantly, this highlights the issue of interpreting patients’ results based on direct comparison to single samples. For example, a patient who is evaluated but doesn’t have intrinsic T cell defects and has a normal SI can be interpreted as abnormal if the control is in the upper range; hence, the patient SI might be <50% of the control. This may lead to a false positive result. The opposite is also possible, where if the control SI is very low, a patient with an intrinsic T cell defect but an SI above 50% of that control might be interpreted as normal, which could result in a false negative result.
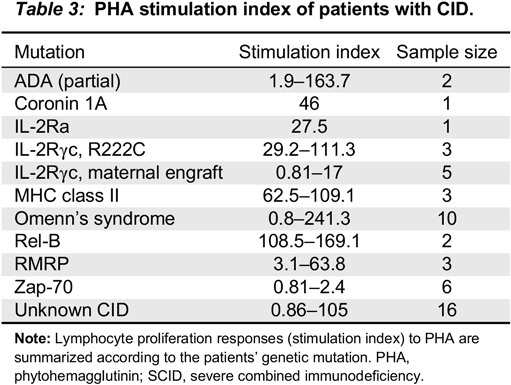
There are groups of CIDs that could have normal lymphocyte enumeration, for example NEMO mutation with immunodeficiency (
Orange et al. 2004), R222C mutations in IL-2Rγc (
Somech and Roifman 2005), and Rel-B deficiency (
Merico et al. 2015), but abnormal mitogen proliferation results. This test therefore becomes important in increasing the suspicion of CID. Furthermore, the results of this study help to establish the SI of healthy controls and assists in the critical interpretation of control values, especially when their SI is at the extreme end of the “normal” range.
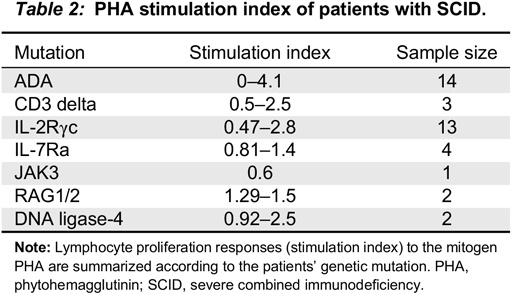
In our cohort, all the patients with typical SCID had profound low proliferative responses, with SI <5, consistent with previous studies (
Buckley et al. 1997;
Roifman et al. 2012). Patients with non-typical SCID, Omenn’s syndrome or patients with maternal T cell engraftment had very low SI, yet somewhat higher than typical SCID (SI 0–30).
Remarkably, almost all patients with CID (98%) had SI of <200 and almost 85% had SI <100. Patients with Zap-70 deficiency (
n = 6) had profoundly low mitogen responses, indistinguishable from SCID. The 2 patients with partial ADA had wide differences in their mitogen response. The first patient had a SI of 1.9 while the second had a SI of 163. PHA responses in cases with cartilage hair hypoplasia were variable, ranging from 5 to 100. Patients with MHC class II deficiency had SI well within the range of CID, unlike previous publications suggesting that these might have normal mitogen responses, but absent antigen responses (
Al-Mousa et al. 2010). This is a good example of the advantage of using our analysis to define normal ranges rather than assaying single samples. The lower SI in MHC class II deficiency might be related to the decreased numbers of CD4+ cells, which contribute to about two-thirds of the T cell population as well as disturbed co-stimulatory molecules that play an important role in mitogen proliferation (
Bonilla 2008). In a similar manner, patients with CID due to Rel-B, CD25, coronin 1A and others have all consistently had PHA levels below 200.
It is notable that some CIDs may present with normal proliferative responses against PHA, but not other mitogens, such as CD3/CD28. For example, loss-of-function mutations in caspase recruitment domain family, member 11 (
Stepensky et al. 2013) result in aberrant NFκB signaling and hypogammaglobulinemia. However, PHA proliferative responses are preserved. Thus, it should be kept in mind that no single laboratory test can identify or define impaired cellular immunity, and all tests should be interpreted with due caution and correlated with clinical context.
Recently, alternative techniques to assess T cell proliferation have been offered. One method is to track lymphocyte division using the fluorescent dye carboxyfluorescein diacetate succinimidyl ester (CFSE), developed by
Lyons and Parish (1994). With this method, the CFSE dye is diluted by half with each cell division until it becomes undetectable (
Quah et al. 2007). Another method uses 5-ethynyl-2′-deoxyuridine (EdU), a fluorescent nucleoside analogue, which directly measures de novo DNA synthesis. EdU incorporates into the DNA of dividing cells using click chemistry and the fluorescence emitted serves as an index of cell proliferation (
Yu et al. 2009). The latter method seems to correlate better to
3H-thymidine when studied in mice (
Yu et al. 2009). The disadvantages of these assays include increased cost, dependence on expensive and complex equipment, and more importantly, the lack of proper control ranges for CID. Studies that compare flow cytometry based assays with
3H-thymidine will aid in defining the advantages and limitations of these assays.