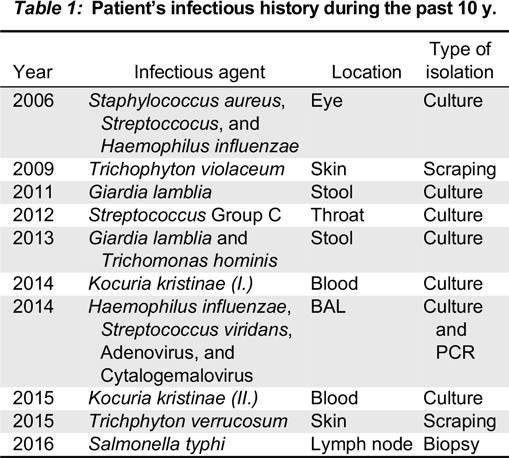
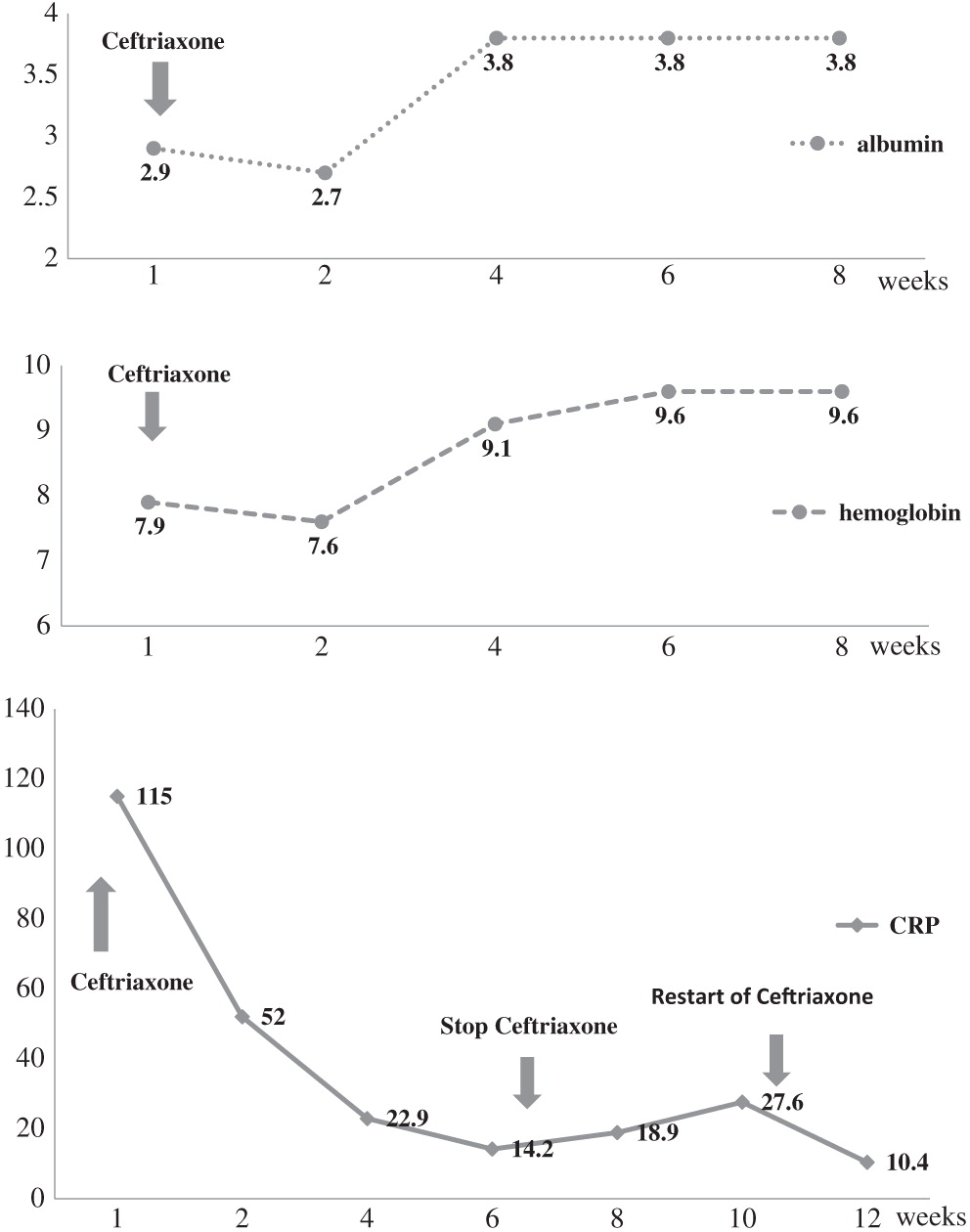
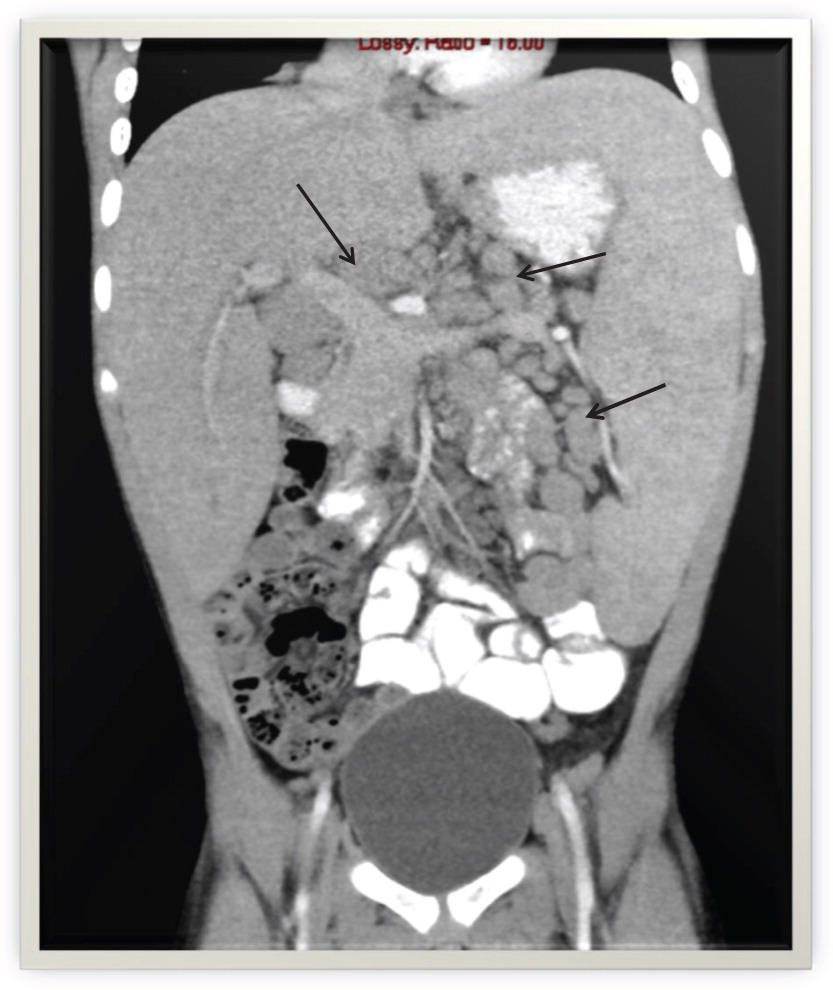
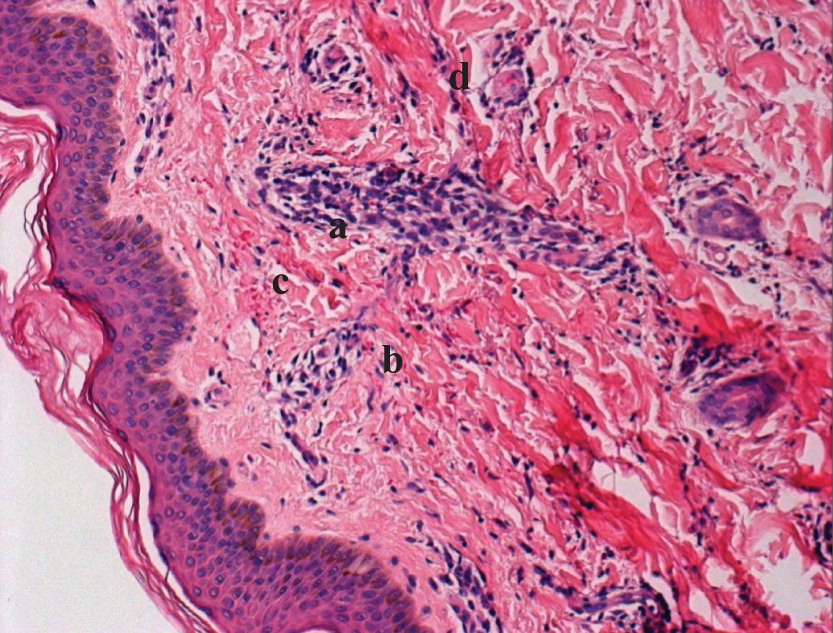
de Beaucoudrey L., Samarina A., Bustamante J., Cobat A., Boisson-Dupuis S., Feinberg J., Al-Muhsen S., Jannière L., Rose Y., de Suremain M., Kong X.F., Filipe-Santos O., Chapgier A., Picard C., Fischer A., Dogu F., Ikinciogullari A., Tanir G., Al-Hajjar S., Al-Jumaah S., Frayha H.H., AlSum Z., Al-Ajaji S., Alangari A., Al-Ghonaium A., Adimi P., Mansouri D., Ben-Mustapha I., Yancoski J., Garty B.Z., Rodriguez-Gallego C., Caragol I., Kutukculer N., Kumararatne D.S., Patel S., Doffinger R., Exley A., Jeppsson O., Reichenbach J., Nadal D., Boyko Y., Pietrucha B., Anderson S., Levin M., Schandené L., Schepers K., Efira A., Mascart F., Matsuoka M., Sakai T., Siegrist C.A., Frecerova K., Blüetters-Sawatzki R., Bernhöft J., Freihorst J., Baumann U., Richter D., Haerynck F., De Baets F., Novelli V., Lammas D., Vermylen C., Tuerlinckx D., Nieuwhof C., Pac M., Haas W.H., Müller-Fleckenstein I., Fleckenstein B., Levy J., Raj R., Cohen A.C., Lewis D.B., Holland S.M., Yang K.D., Wang X., Wang X., Jiang L., Yang X., Zhu C., Xie Y., Lee P.P., Chan K.W., Chen T.X., Castro G., Natera I., Codoceo A., King A., Bezrodnik L., Di Giovani D., Gaillard M.I., de Moraes-Vasconcelos D., Grumach A.S., da Silva Duarte A.J., Aldana R., Espinosa-Rosales F.J., Bejaoui M., Bousfiha A.A., Baghdadi J.E., Ozbek N., Aksu G., Keser M., Somer A., Hatipoglu N., Aydogmus C., Asilsoy S., Camcioglu Y., Gülle S., Ozgur T.T., Ozen M., Oleastro M., Bernasconi A., Mamishi S., Parvaneh N., Rosenzweig S., Barbouche R., Pedraza S., Lau Y.L., Ehlayel M.S., Fieschi C., Abel L., Sanal O., and Casanova J.L. 2010. Revisiting human IL12 R B1 deficiency: A survey of 141 patients from 30 countries. Medicine (Baltimore).89:381–402.