Patient 2
Patient 2 was born at 33 weeks of gestation by cesarean section due to IUGR and oligohydramnios, with a birth weight of 1.3 kg. He is the first child of non-consanguineous Caucasian parents, with no history of previous miscarriages. The mother was noted to have hypothyroidism, eczema, and vaginal candidiasis, and the father had hyperthyroidism and pityriasis rosea. A family history of early death in childhood due to pneumonia, in 2 siblings of the maternal grandfather, was reported.
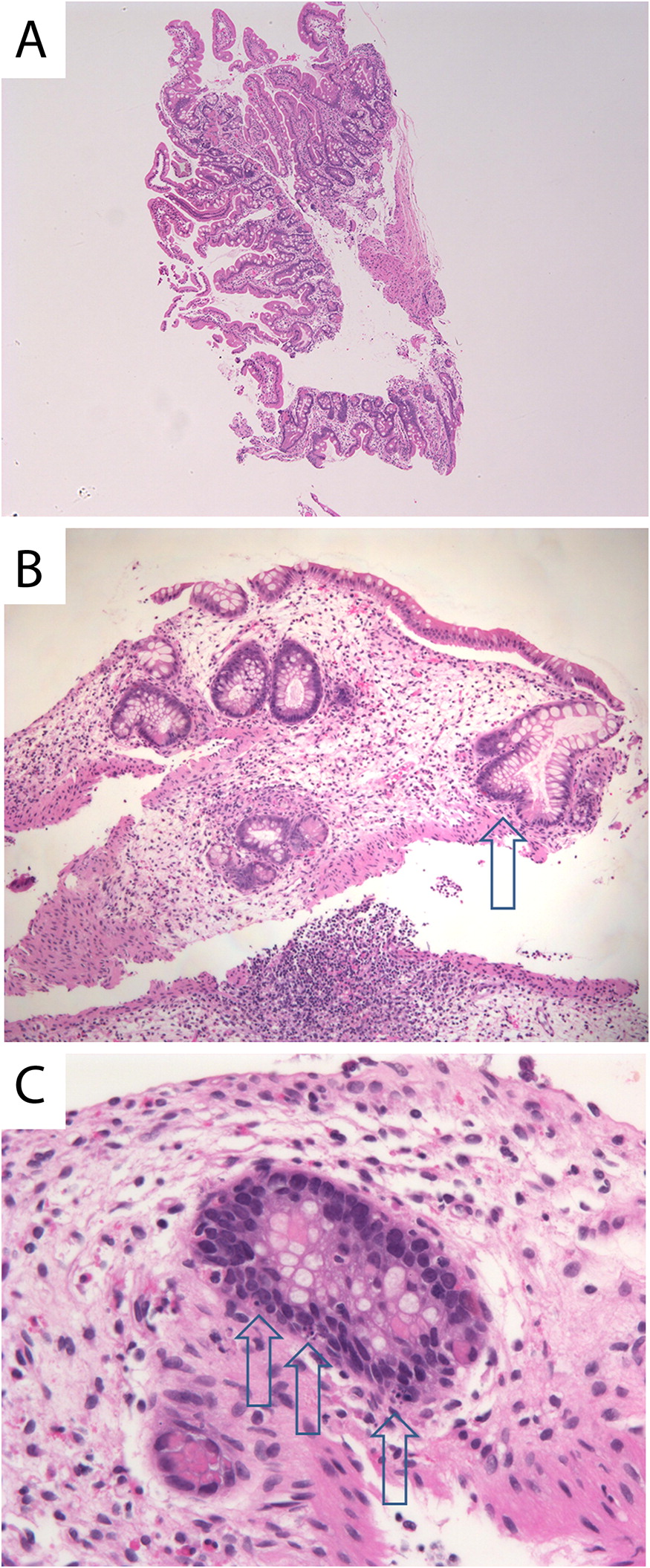
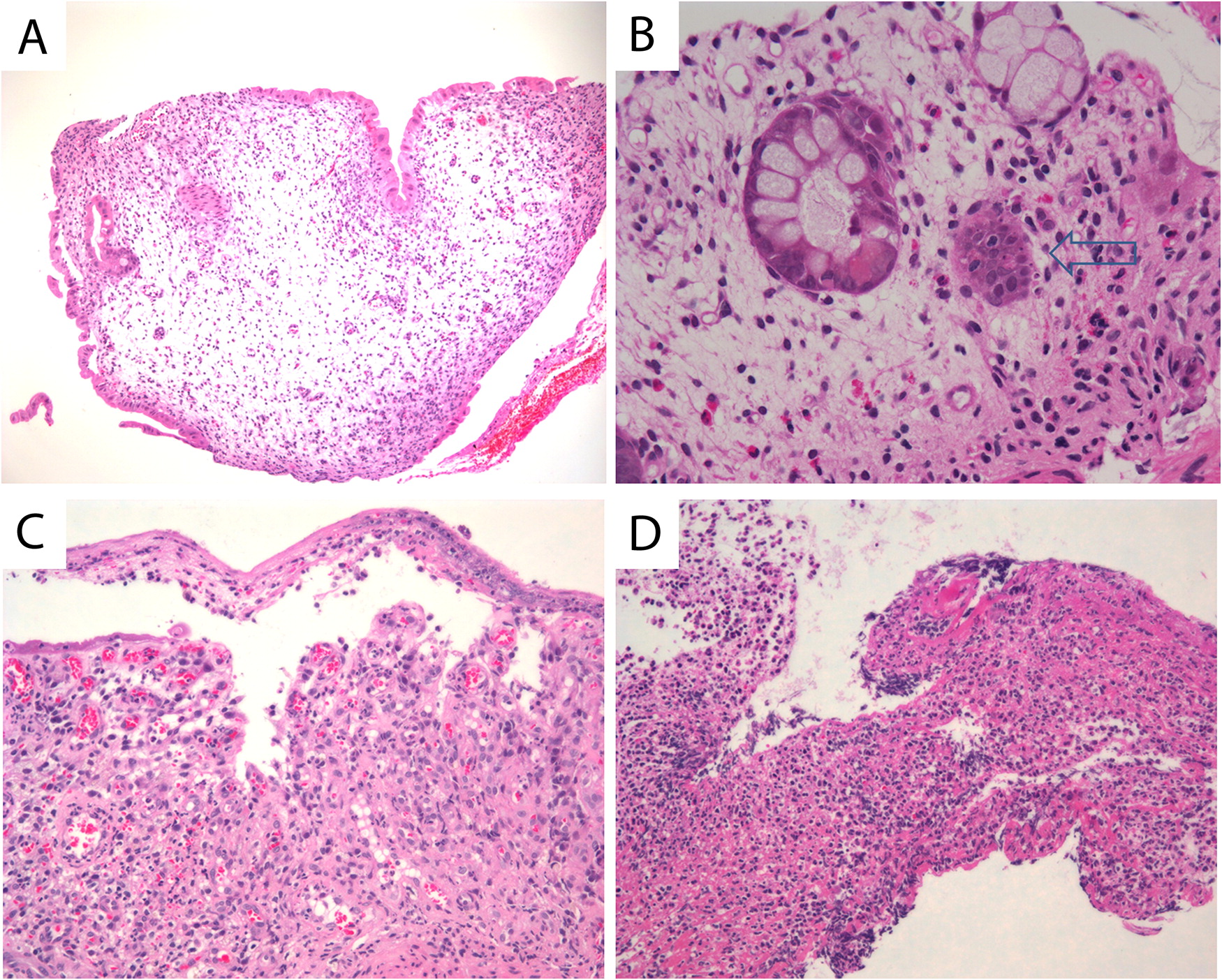
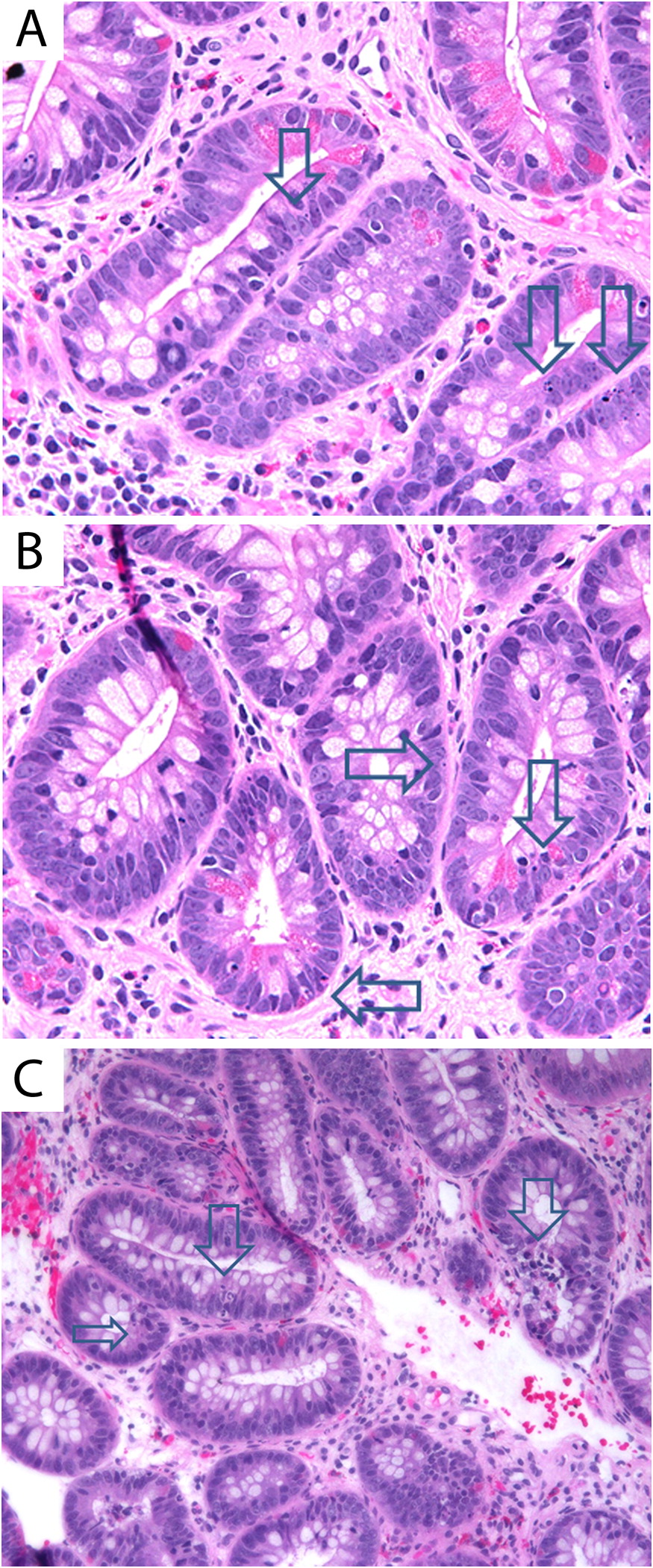
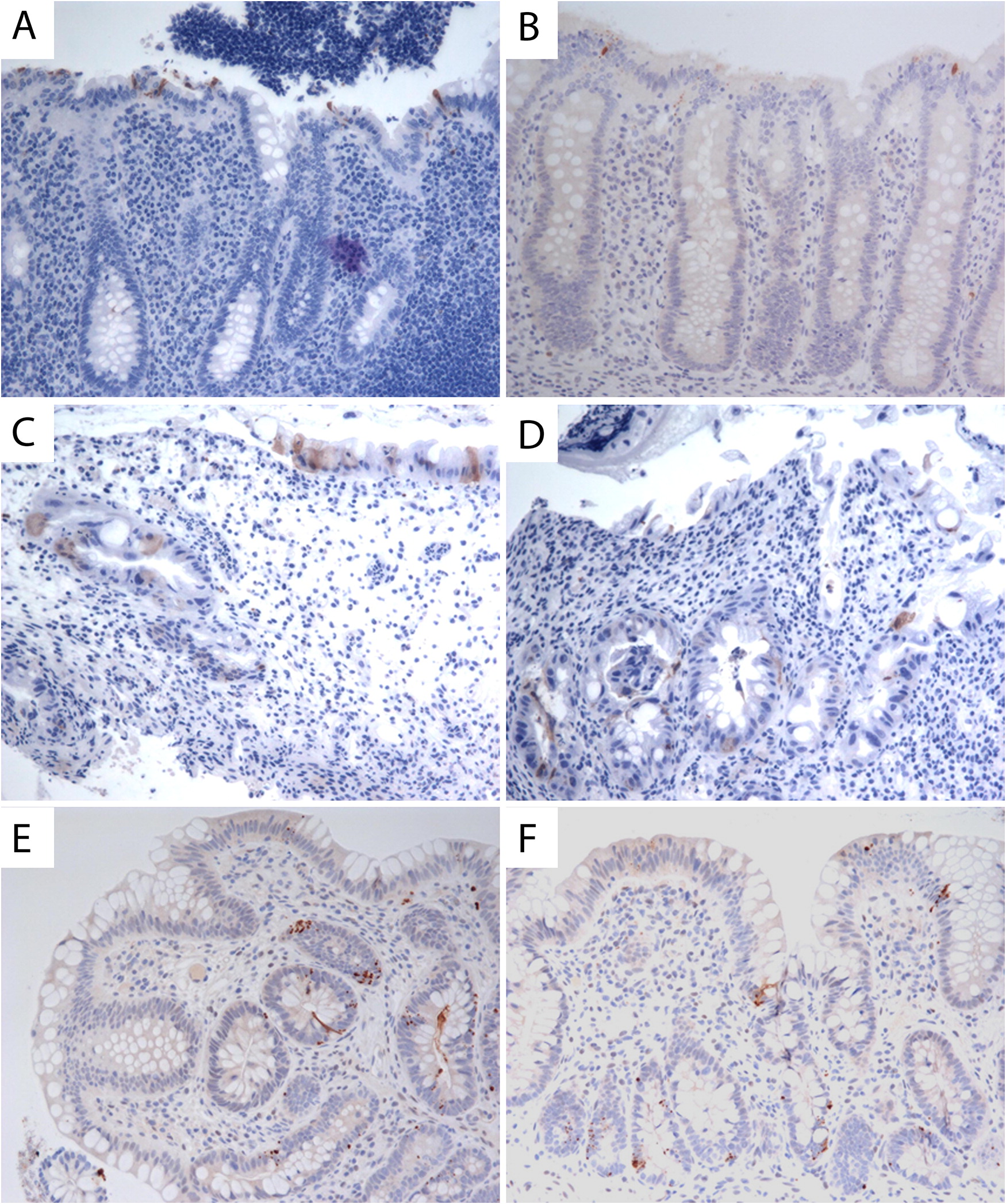
After birth, the patient had a prolonged 5-week course of hospitalization for feeding difficulties. At 14 months of age, he was noted to have a white plaque on the tongue that was not responsive to antiviral, antifungal, or corticosteroid treatment. A biopsy of the lesion demonstrated scar tissue. He also experienced chronic bloody diarrhea and reduced appetite, followed by weight loss and failure to thrive (FTT).
At the age of 3 years, an upper and lower gastrointestinal endoscopy was performed, revealing white esophageal plaques and severe patchy colitis involving the rectum and proximal sigmoid. An esophageal biopsy demonstrated superficial epithelial invasion of Candida hyphae. Systemic antifungal treatment was administered for the esophageal lesions, resulting in partial improvement of his symptoms. His height and weight were both below the third percentile for age and he also had leukoplakia. The patient was developmentally appropriate for age. Immune evaluation demonstrated a normal white blood count of 7.8 (5.0–12.0 × 109/L), mild anemia with hemoglobin levels of 102 (110–10 g/L), and normal platelet levels of 186 (150–400 × 109/L). Neutrophil and lymphocyte counts were normal at 4.25 (1.5–8.5 × 109/L) and 2.34 (2–8 × 109/L), respectively. Monocyte counts were mildly increased at 1.03 (0.05–0.80 × 109/L). Liver enzymes, albumin, total protein, electrolytes, and kidney function were all normal. His IgG level was in the lower range of normal 4.9 (4.5–14.3 g/L), whereas other immunoglobulin levels, including IgE, were normal. Lymphocyte immunophenotyping revealed a borderline low count of CD19 cells at 213 (200–2100 cells/μL) and low count of NK cells at 78 (100–1000 cells/μL). CD4 counts were normal at 1342 (500–2400 cells/μL), CD8 of 826 (300–166 cells/μL), total CD3 of 2371 (900–4500 cells/μL) and normal CD4 to CD8 ratio of 1.6 (0.9–2.9). He had a good immune response to the measles, rubella, and tetanus vaccine and an equivocal antibody titer to mumps and varicella. Isohemagglutinins were poor with anti-A 1:4 and anti-B 1:1. Neutrophil oxidative burst index was normal. In vitro lymphocyte stimulation assay with PHA was robust at 1101 (normal >200). An extended endocrinology evaluation was unremarkable.
Shortly afterwards, the patient developed reticulated hyperpigmented lesions on his neck, axilla, and groin, and nail changes with vertical ridging and lichen planus appearance. Together with the ongoing colitis, FTT, and leukoplakia, these new findings were suggestive of dyskeratosis congenita. Genetic testing confirmed a mutation at the DKC1 gene c.146 C>T predicting an amino acid change of threonine to methionine at position 49 (T49M). This mutation was previously reported to cause HHS (
Knight et al. 1999).
Since the diagnosis, the patient continues to demonstrate significant FTT with microcephaly (head circumference is 2–3 standard deviations below mean) and intermittent colitis. Surprisingly, he has no history of recurrent significant infections. Repeat bone marrow biopsies and aspirations demonstrate mild hypocellularity. Ongoing immune evaluations demonstrate normal humoral and cellular function, despite hypogammaglobinemia with low IgG level of 4.8 (5.4–13.6 g/L) and low counts of B and NK cells.