Background
In 1859 Louis Pasteur proposed that infectious diseases were caused by germs (
Ullmann 2007). In 1876, Robert Koch proved Pasteur’s germ theory by isolating the anthrax bacillus and later the turbercle bacillus (
Encyclopedia Britannica 2014). Koch also set up a bacteriology laboratory that attracted scientists from around the world and resulted in the development of new methods to isolate and study pathogenic bacteria.
Koch formulated 4 basic criteria to prove that a disease was caused by a specific microorganism (
Encyclopedia Britannica 2014):
1.
“A specific microorganism is always associated with a given disease.”
2.
“The microorganism can be isolated from the diseased animal and grown in pure culture in the laboratory.”
3.
“The cultured microbe will cause disease when transferred to a healthy animal.”
4.
“The same type of microorganism can be isolated from the newly infected animal.”
Koch and his collaborators contributed directly to the development of antibody therapy. While studying in Koch’s laboratory, the Japanese scientist Shibasaburo Kitasato produced the first pure culture of the anaerobic bacterium
Clostridium tetani (
Kitasato 1889) and discovered the existence of tetanus toxin in the filtrates of
C. tetani cultures (
von Behring and Kitasato 1890). These studies were published in the landmark paper entitled “On the mechanism of immunity to diphtheria and tetanus in animals” (
von Behring and Kitasato 1890). In this article, the authors described the following experiment: (
i) rabbits were vaccinated with a culture of virulent tetanus bacteria; (
ii) blood was collected from the immunized rabbits; and (
iii) 0.2–0.5 mL of the immune plasma injected into the abdominal cavity of mice protected them against lethal doses of
C. tetani.
Kitasato and von Behring summarized their observations as follows:
“The blood of rabbits immune to tetanus has the ability to neutralize or destroy the tetanus toxin…This property exists also in extravascular blood and in cell-free serum…This property is so stable that it remains effective even in the body of other animals, so that it is possible, through blood or serum transfusions, to achieve an outstanding therapeutic effect…The property which destroys tetanus toxin does not exist in the blood of animals which are not immune to tetanus, and when one incorporates tetanus toxin into nonimmune animals, the toxin can still be demonstrated in the blood and other body fluids of the animal, even after its death.”
One week after the tetanus data were published,
von Behring (1890) reported similar results with diphtheria bacteria in a paper entitled “Studies on the mechanism of immunity to diphtheria in animals”. von Behring and Kitasato were the first to discover antibody molecules in the serum of immunized animals and to demonstrate that these antibodies could neutralize diphtheria and tetanus toxins (
Sri Kantha 1991). They also demonstrated that the antibodies were specific; tetanus antitoxin did not neutralize diphtheria toxin and vice versa (
Sri Kantha 1991).
Antibodies are gamma globulins
Several investigators reported that antibodies were transmitted from mother to infant through the placenta. Investigators also observed that the concentration of diphtheria and tetanus antitoxins in fetal blood were the same as in maternal blood (
McKhann and Chu 1933). Based on these observations,
McKahnn and Chu (1933) studied the distribution of antibodies in the protein fractions of placental extracts. Extracts of placentas were fractionated by ammonium sulfate precipitation and tested for diphtheria anti-toxin. The globulin fractions contained diphtheria toxin-neutralizing activity (
McKhann and Chu 1933).
McKahnn and Chu (1933) also reported that placental extracts given by intramuscular injection early after exposure to measles “either completely protects children against measles or produces a modification of the disease”.
Further characterization of antibody molecules was provided by physical chemists in Sweden.
Heidelberger and Pederson (1937) separated antibody proteins on the basis of their molecular weights by sedimentation in an ultracentrifuge. Using material provided by sedimentation,
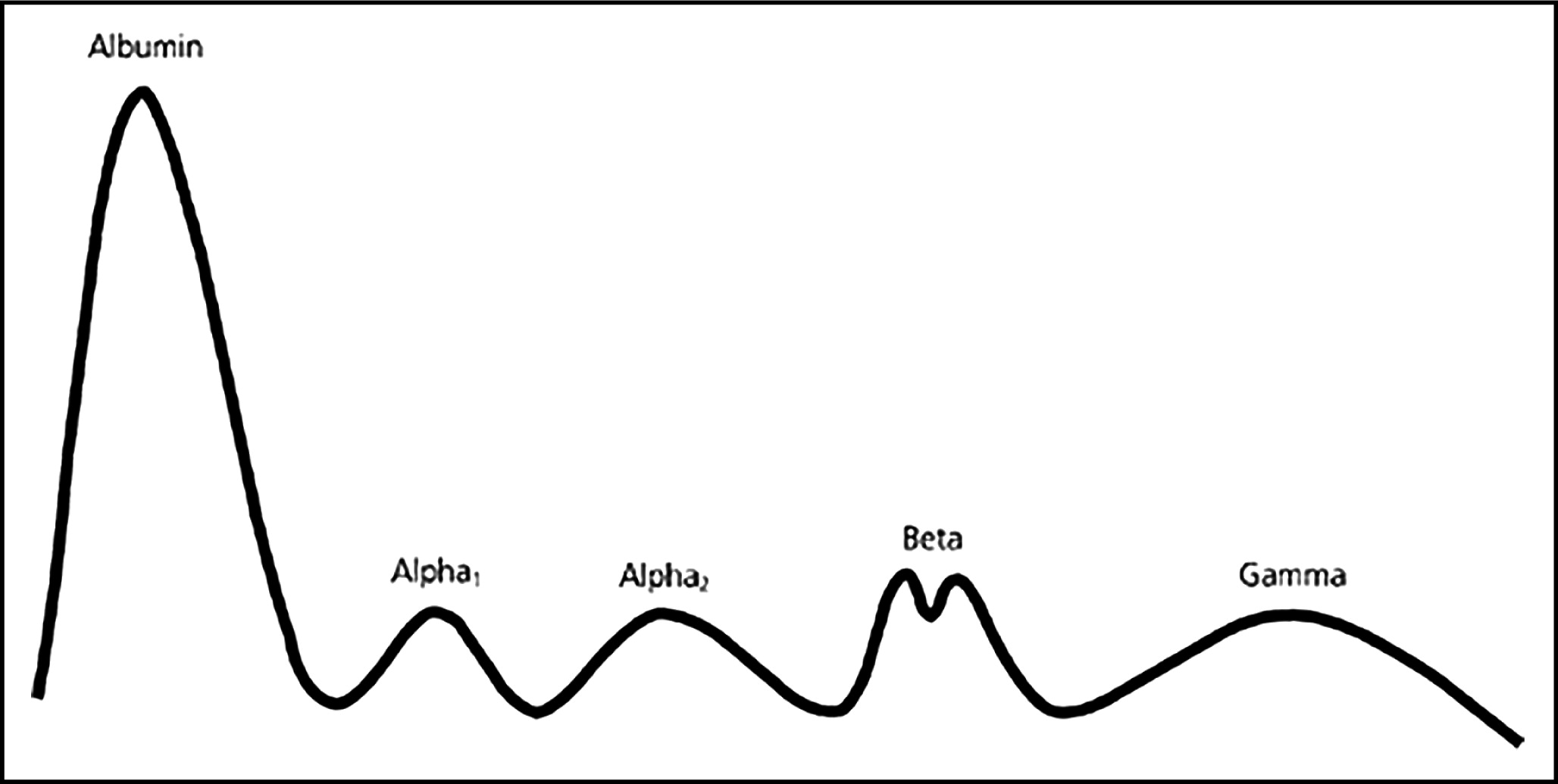
Tiselius (1937) further separated the globulins by their electrical charge using electrophoresis. Antibodies were found in the most slowly migrating globulin fraction, which was designated the “gamma globulin” fraction (
Tiselius 1937). In further studies
Tiselius and Kabat (1939) published an electrophoretic profile of the serum proteins as illustrated in
Fig. 1.
Immune serum globulin (an antibody concentrate)
The purification of serum globulins from human plasma by
Cohn et al. (1944) provided material that could be safely injected into patients.
Cohn et al. (1944) developed a series of cold ethanol precipitation steps that fractionated human plasma into classes of proteins, including the gamma globulin fraction commonly known as Cohn fraction II.
Enders analyzed fraction II for antibody activities and showed that 5 antibodies, including diphtheria antitoxin and mumps antibody, were concentrated 15–30 times compared with their concentration in the starting plasma (
Enders 1944).
Stokes et al. (1944) demonstrated that immune serum globulin (ISG), formulated from Cohn fraction II at 165 mg/mL protein, protected or attenuated infections in children exposed to measles during epidemics in the winter of 1942 and the spring of 1943. They observed that almost all children 5 years of age and under were protected by doses of 2.0–2.5 mL of ISG injected intramuscularly, whereas complete protection of children 6–12 years of age required 4–5 mL of ISG.
Stokes et al. (1944) also observed that some patients who had already developed symptoms of disease benefited from higher doses of ISG, and speculated that high doses delivered intravenously might provide therapeutic value in the early stages of measles infection.
In 1952, Bruton treated a child with undetectable serum gamma globulin levels who suffered from recurring pneumococcal infections. Subcutaneous infusions of 3.2 g/month ISG produced measureable circulating gamma globulin levels and completely eliminated the pneumococcal infections. These observations were confirmed by investigators in the United States who reported on 9 patients (
Janeway et al. 1953) and in a report of 176 patients in the United Kingdom (
Medical Research Council 1969).
Shown to be effective for post-exposure prophylaxis of measles and hepatitis A infections, ISG became the standard of care for patients with primary antibody deficiencies (
Medical Research Council 1969;
Schiff 1994). The standard dose was approximately 100–150 mg/kg. When children with measles infections were given ISG intravenously, they suffered from severe adverse reactions including fever, convulsions, restlessness, chills, and vasomotor collapse (
Schiff 1994). Therefore, ISG administration was restricted to intramuscular or subcutaneous injection.
Intravenous immunoglobulin (IVIG)
The desire to deliver larger doses of ISG led to a series of manufacturing changes intended to produce an intravenously injectable immunoglobulin. Early IVIG preparations were produced from ISG by digestion with proteolytic enzymes to eliminate anticomplement activity. Anticomplement activity was believed to be the primary cause of adverse side-reactions when ISG was injected intravenously (
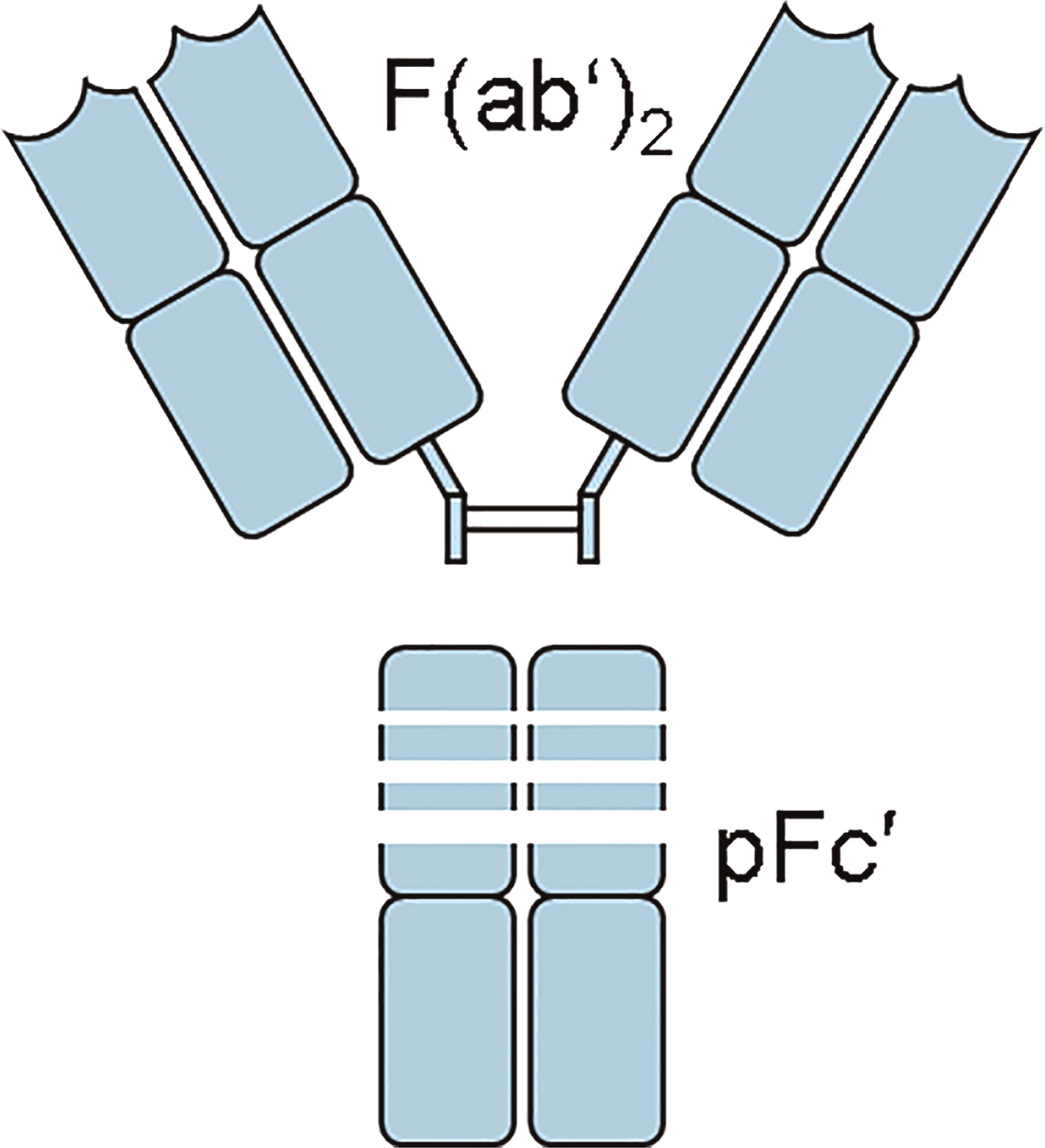
Barandun et al. 1962). The first commercial IVIG was produced by digestion of ISG with pepsin. The principal component of pepsin-digested IVIG was a fragment that contained 2 antibody binding sites and was named the F(ab′)2 fragment (
Fig. 2) (
Schultze and Schwick 1962).
In addition to manufacturing procedures, IVIG formulations have also changed over the years. Historically, most commercial IVIGs were formulated at pH 6.4–7.2 and 50 mg/mL. These products were usually freeze-dried in the presence of a stabilizer because IgG solutions at neutral pH are unstable and form precipitates during storage. The discovery that IgG solutions at low pH are physically stable has resulted in current IVIG products being formulated as liquids.
IVIG in the United States
The first IVIG licensed in the United States was produced from Cohn fraction II by reduction and alkylation (Schroeder et al. 1980). Using this product (Gamimune),
Nolte et al. (1979) randomized 20 patients with primary immunodeficiency (PID) to receive IVIG or ISG. Doses were 150 mg/kg every 4 weeks of IVIG or 3.3 g/4 weeks of ISG. Since many subjects had chronic otitis media, sinusitis, and bronchitis, these acute infections, defined by “a spiking fever greater than 100.4 °F with or without chills, clinical evidence of a purulent infection, and positive cultures”, were monitored. The authors observed fewer acute infections during IVIG treatment but noted that the IVIG dose was significantly higher than the ISG dose (
Nolte et al. 1979). A similar observation was made by
Roifman et al. (1985).
The same product was studied by
Amman et al. (1982) in a randomized 2-year crossover study. Thirty-four patients with serum IgG levels <300 mg/dL and who were unresponsive to immunization were studied. The patients were treated for 1 year with either IVIG or ISG at a dose of 100 mg/kg per month and then were treated for an additional year with the other product. No differences were observed in the incidence of acute infections but upper respiratory illnesses were more prevalent in IVIG recipients (85.3% vs 50.0% of patients,
p < 0.006). This suggested that the dose rather than the rate of administration might be critical.
In a cross-over study of 12 patients with antibody deficiency and lung disease,
Roifman et al. (1987) studied the impact of IVIG dose on clinical outcomes. Patients received either 600 mg/kg or 200 mg/kg of IVIG for 6 months and then were switched to the other dose for 6 months. The incidence of acute infections was substantially lower in patients receiving the higher dose when their serum IgG level reached ≥500 mg/dL. Lung function consistently improved in high dose recipients (
Roifman et al. 1987).
In another crossover study, 12 PID patients received 500 mg/kg per month during the first year and 150 mg/kg per month during the second year, or vice versa. Fewer days of infection and less antibiotic treatment were required during high dose therapy. Compared with low-dose IVIG, high-dose IVIG reduced the number of fever days and improved lung function in some patients (
Bernatowska et al. 1987).
A wide range of doses have been used in IVIG clinical trials of patients with PID. Most studies have shown a significant benefit when immunodeficient patients are treated with doses of 300 mg/kg per month or more (
Schiff 1994).
IVIG in PID
All IVIGs licensed in the United States are studied in patients with PID. Early studies involved relatively few patients, several different doses, and different outcome parameters. In 1999, the US Food and Drug Administration (FDA) attempted to standardize IVIG clinical trials and proposed that new IVIGs be studied in a randomized, double-blind, non-inferiority study of 80 subjects with PID in which the safety and efficacy of the test product was to be compared with a US-licensed IVIG product over an observation period of 12 months (
FDA Blood Products Advisory Committee Meeting, March 1999).
Subsequently, the FDA proposed that testing smaller numbers of subjects in an open-label, single-arm trial compared with a statistically modelled historical control would be sufficient to provide evidence of safety and efficacy (
FDA Blood Products Advisory Committee Meeting, March 2000). In 2005, the FDA guidance document suggested that 40–50 subjects was an adequate number of patients, and safety and efficacy criteria were also defined (
FDA/CBER Guidance for Industry 2005).
This guidance defined “infusional adverse events” (AE) as a safety parameter. Infusional AE are temporally associated with an infusion and occur during or within 1 h, 24 h, or 48 h following infusion of the test product. A principal safety endpoint was the observed proportion of infusions with 1 or more temporally associated AEs (including AEs that were determined not to be product related). The target for this endpoint was an upper one-sided 95% confidence limit of <0.40 (
FDA/CBER Guidance for Industry 2005). The most recent FDA Guidance published in 2008 extended the time period for infusional AEs to 72 h and added instructions for conducting pharmacokinetic studies in pediatric patients (
FDA/CBER Guidance for Industry 2008).
The principal efficacy parameter was defined as the rate of serious bacterial infections in adult and pediatric subjects over a period of 12 months (to avoid seasonal biases). The efficacy target was a statistical demonstration that the upper one-sided 99% confidence limit of serious bacterial infections is <1.0 per person per year. The FDA guidance documents defined the types of serious bacterial infections and the diagnostic criteria for each type.
The effect of these FDA guidance documents has been to standardize IVIG clinical trials in patients with PID in the United States.
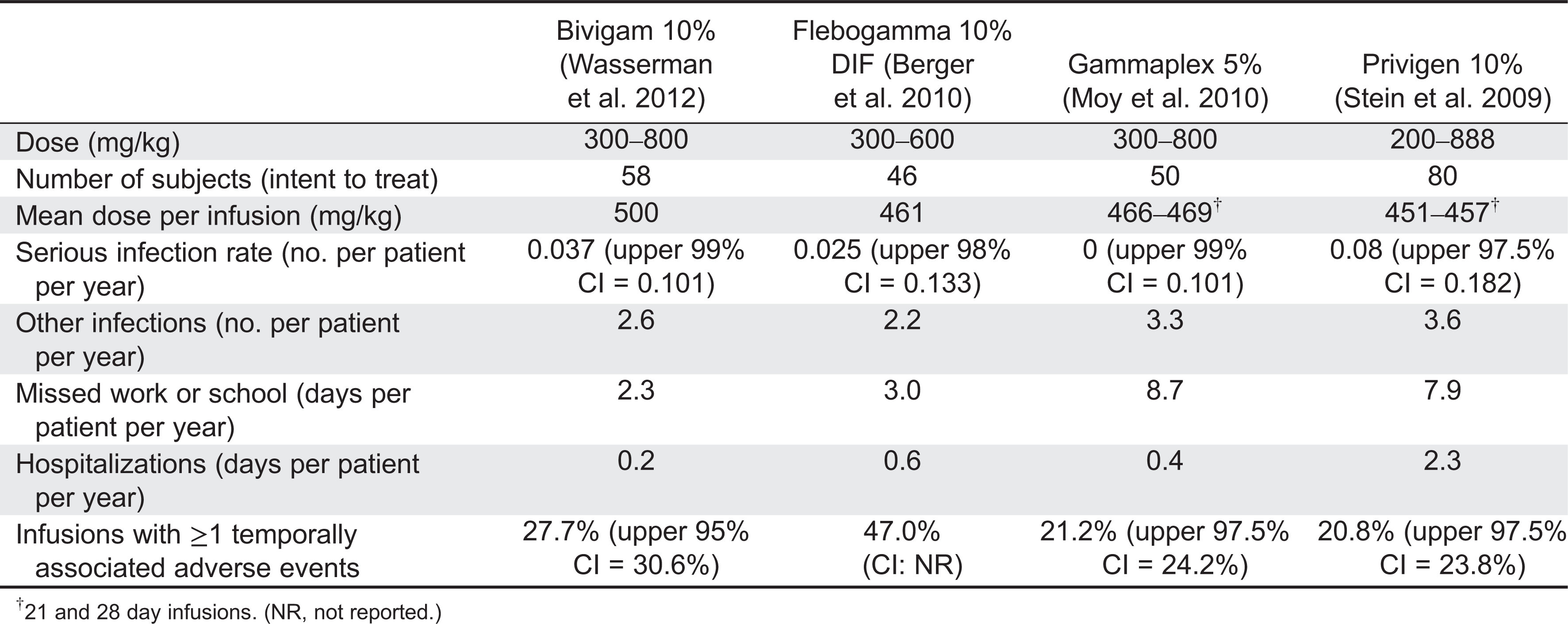
Table 1 summarizes results from recent IVIG clinical trials (
Stein et al. 2009;
Berger et al. 2010;
Moy et al. 2010;
Wasserman et al. 2012). The number of patients in each trial ranged from 46 to 80 and the mean dose per patient was 461–500 mg/kg. The same serious bacterial infection and adverse event endpoints were reported for each study.
Subcutaneous immunoglobulin therapy
Berger et al. (1980) published a paper on the use of small pumps to infuse patients with ISG subcutaneously at rates of 1–2 mL/h instead of by intramuscular injection. Termed “slow subcutaneous infusions,” the infusions were accompanied with little swelling or pain at the infusion site and no systemic reactions (
Berger et al. 1980).
Subcutaneous infusions of immunoglobulin have become a popular mode of delivery in patients with primary immunodeficiency.
Gardulf et al. (1991) reported on a clinical trial of rapid subcutaneous immunoglobulin infusions in 25 consecutive patients with hypogammaglobulinemia who had been previously treated with intramuscular ISG injections. Patients were infused at 34–40 mL/h with 100 mg/kg per week with a mercury-free ISG (produced by Kabi) delivered in divided doses at 2–6 infusion sites on the abdominal wall and (or) the thigh. Patients were trained at the study site to infuse themselves and after 6 months of treatment received their remaining infusions at home. The authors reported that the subjects experienced fewer adverse reactions compared with ISG treatment (
Gardulf et al. 1991). Since this article was published, there have been many follow-up publications by the same authors and subcutaneous immunoglobulin therapy has become a standard treatment for patients with immunodeficiency in Scandinavian countries (
Lingman-Framme and Fasth 2013).
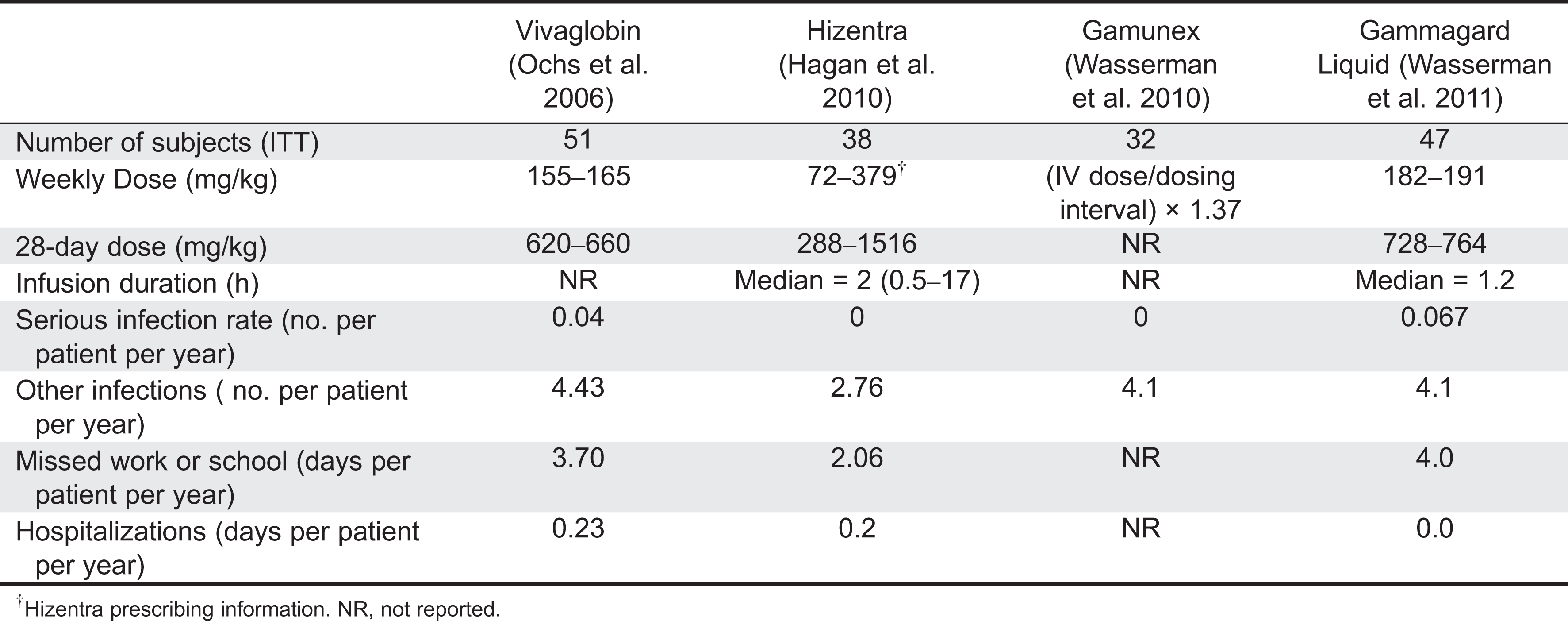
In 2006, the safety and efficacy of a mercury-free ISG preparation (Vivaglobin, CSL Behring) was studied in an open-label multicentre study in PID patients in the United States (
Ochs et al. 2006). Sixty-five patients previously treated with IVIG were enrolled in a 3-month SCIG treatment period. Fifty-one of these patients were then treated for 12 months with weekly SCIG infusions at a dose calculated to be equal to 137% of their previous IVIG dose. This resulted in a mean weekly dose of 158 mg/kg per week with a range of 155–165 mg/kg per week. Trough IgG levels increased from 786 mg/dL at baseline to 1040 mg/dL during SCIG treatment. The first 2 subcutaneous infusions were administered under the supervision of trained nurses. SCIG was administered at 1 or more injection sites using an infusion pump. Infusion rates were ≤ 20 mL/h at any single injection site and the volume per site was ≤15 mL (
Ochs et al. 2006).
During the 12-month efficacy phase, 2 serious bacterial infections (SBI) were reported, both pneumonias, for an incidence of 0.04 SBI per patient per year. The annual rate of all infections was 4.43 per patient per year and the number of days with infection was 118 per patient-year. The most frequent AE was infusion site reaction, which occurred at least once in 60 patients (91%). The next most frequent AE was headache. Thirty-one subjects (48%) reported a total of 159 episodes of headache during the 12-month efficacy period (
Ochs et al. 2006).
The same manufacturer recently licensed another SCIG known by the trade name Hizentra®. Hizentra is a 20% (
w/
v) IgG solution that was studied in an open-label, multicentre, single-arm study of safety and efficacy (
Hagan et al. 2010). Initially, 38 patients were treated for 12 weeks with a weekly dose that was calculated from the last 3 IVIG infusions received prior to the study and multiplied by 1.30 as reported previously (
Ochs et al. 2006). The mean dose during this phase ranged from 176.8 to 182.9 mg/kg per week. During the efficacy period that followed, 23 subjects received 54 SCIG infusions, and 15 subjects received from 11 to 53 SCIG infusions at mean doses of 179.6–224.3 mg/kg per week (
Hagan et al. 2010). The median IgG trough level during this period was 12.5 ± 3.2 mg/mL.
There were no serious bacterial infections during the study. There were 96 nonserious bacterial infections in 31 patients. Sinusitis was the most common infection followed by nasopharyngitis. Twelve patients missed 71 days of work or school (
Hagan et al. 2010). All 49 patients experienced local AEs. Excluding local reactions, 45 patients experienced at least 1 AE and 25 patients had an AE that was considered to be related to the study product (
Hagan et al. 2010). Results of these studies are listed in
Table 4.
Two IVIG preparations have been approved for subcutaneous (SC) administration: Gamunex and Gammagard Liquid. Gamunex was studied in an open-label pharmacokinetic trial to compare the steady state area under the concentration–time curve (AUC) of subcutaneous infusions compared with the AUC obtained from intravenous infusions (
Wasserman et al. 2010). All patients received IV infusions of Gamunex for 3–4 months prior to being switched to weekly SC Gamunex infusions. The SC dose was determined by dividing the IV dose by the dosing interval (3 or 4 weeks) and multiplying by 1.37. The mean adjusted AUC
0-t values for SC and IV administration were 6858 mg·h/mL and 7640 mg·h/mL, respectively. Statistical analysis of these values showed that SC Gamunex was not inferior to IV Gamunex. The mean overall steady-state trough concentration of total plasma IgG was 11.4 mg/mL during SC administration and 9.6 mg/mL following IV administration (
Wasserman et al. 2010). Other results are shown in
Table 4.
Subcutaneous administration of Gammagard Liquid was also studied in an open-label trial of 49 patients with PID (
Wasserman et al. 2011). All subjects received IV infusions of Gammagard Liquid for 13 weeks. One week after the final IV dose, the subjects began to receive weekly subcutaneous doses at 137% of the IV dose. The plasma IgG trough level was 12.0 ± 2.8 mg/mL during SC administration and 10.5 ± 2.60 during IV treatment. AUC values were 9176 ± 1928 days × mg/dL for SC treatment and 9958 ± 2274 days × mg/dL for IV treatment, and were determined to be equivalent. Additional safety and efficacy results are shown in
Table 4.
In summary, SCIG clinical trials have shown relatively high IgG trough levels when compared with IVIG in the same study (
Wasserman 2010,
2011).
SCIG clinical applications
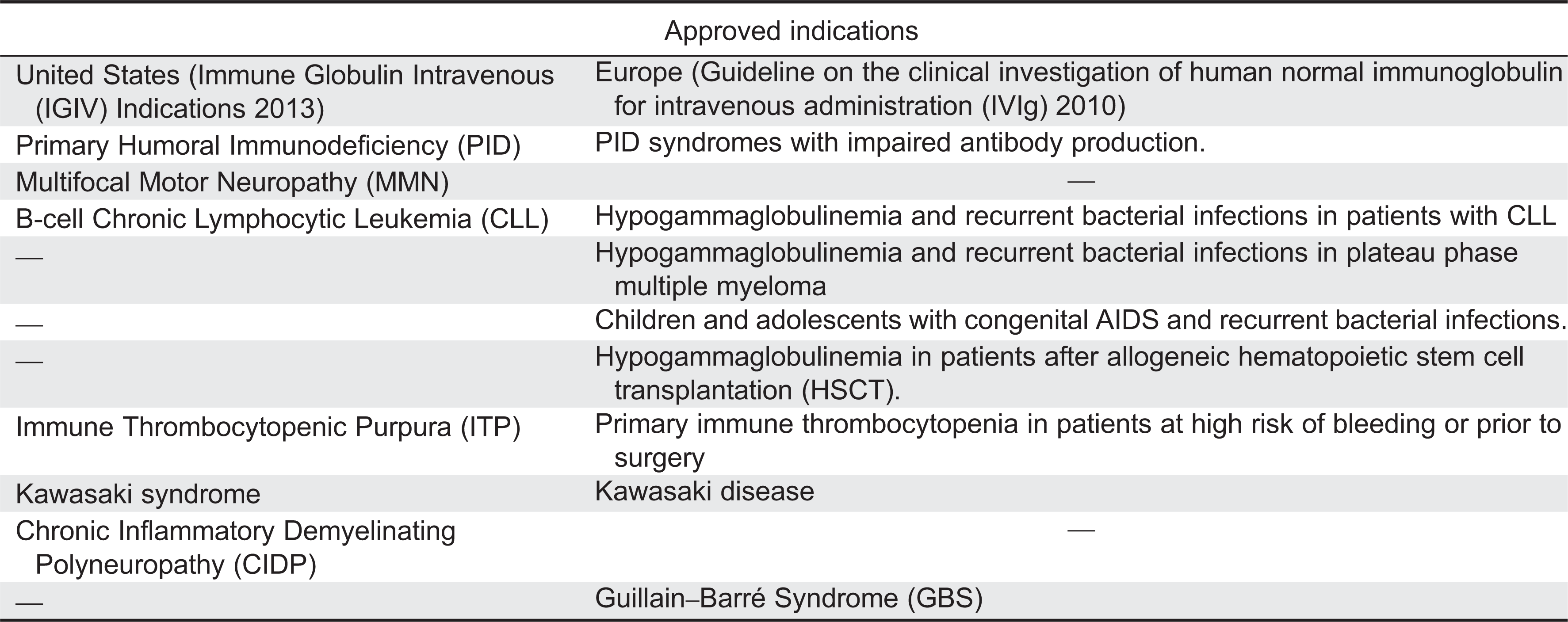
In the United States, primary humoral immunodeficiency is the only approved indication for SCIG and subcutaneously administered IVIG. Other clinical applications have been reported in the literature. In the United Kingdom, SCIG has been used to treat 17 patients with autoimmune neuropathies and 1 patient with Epidermolysis bullosa acquista (
Misbah et al. 2011). A retrospective study of 12 children with prolonged hypogammaglobulinemia after hematopoietic stem cell transplantation found that they experienced fewer side-effects and no more infections that children who received IVIG (
Lingman-Framme and Fasth 2013). SCIG has also been used instead of IVIG in maintenance therapy of patients with MMN and CDIP (
Jolles et al. 2011). SCIG therapy has also been studied as the first immunoglobulin treatment of patients with polymyositis or dermatomyositis (
Jolles et al. 2011).
It is noteworthy that the first patient with primary immunodeficiency treated by
Bruton (1952) was administered immunoglobulin subcutaneously. SCIG has become increasingly popular in the treatment of patients with PID and, as new clinical trials are conducted, may become an alternative to IVIG for other clinical indications.
AEs of immunoglobulins
Clinical trials data for both IVIG and SCIG are relatively standardized with respect to the reporting of AEs since the FDA guidance was issued (
FDA/CBER Guidance for Industry 2008). A review of FDA approved labeling of licensed immunoglobulins indicates that the most common temporally associated (within 72 h) AE associated with immunoglobulins is headache, which occurs at a frequency of 8%–15% of IVIG infusions. Headache is also observed in SCIG recipients at rates of 1.4%–4%. Other adverse events experienced by IVIG recipients are fatigue, fever, and nausea. SCIG recipients experience the same adverse events and also local adverse events such as discomfort and pain, erythema, swelling/edema, and pruritis (
Biotest 2012;
Baxter 2013,
2014;
CSL Behring 2015).