The Institutional Research Ethics Board at The Hospital for Sick Children, Toronto, approved the study and a written informed consent for the research was obtained. The female patient, born to Inuit nonconsanguineous parents, was identified in infancy as suffering from ADA deficiency based on absent ADA activity in the erythrocytes and the identification of compound heterozygous mutations in the ADA gene (c.424C> T and c.955_959delGAAGA). An HLA-matched donor for HSCT could not be found; therefore, the patient was treated with PEG-ADA (ADAGEN®) resulting in a very limited immune reconstitution (
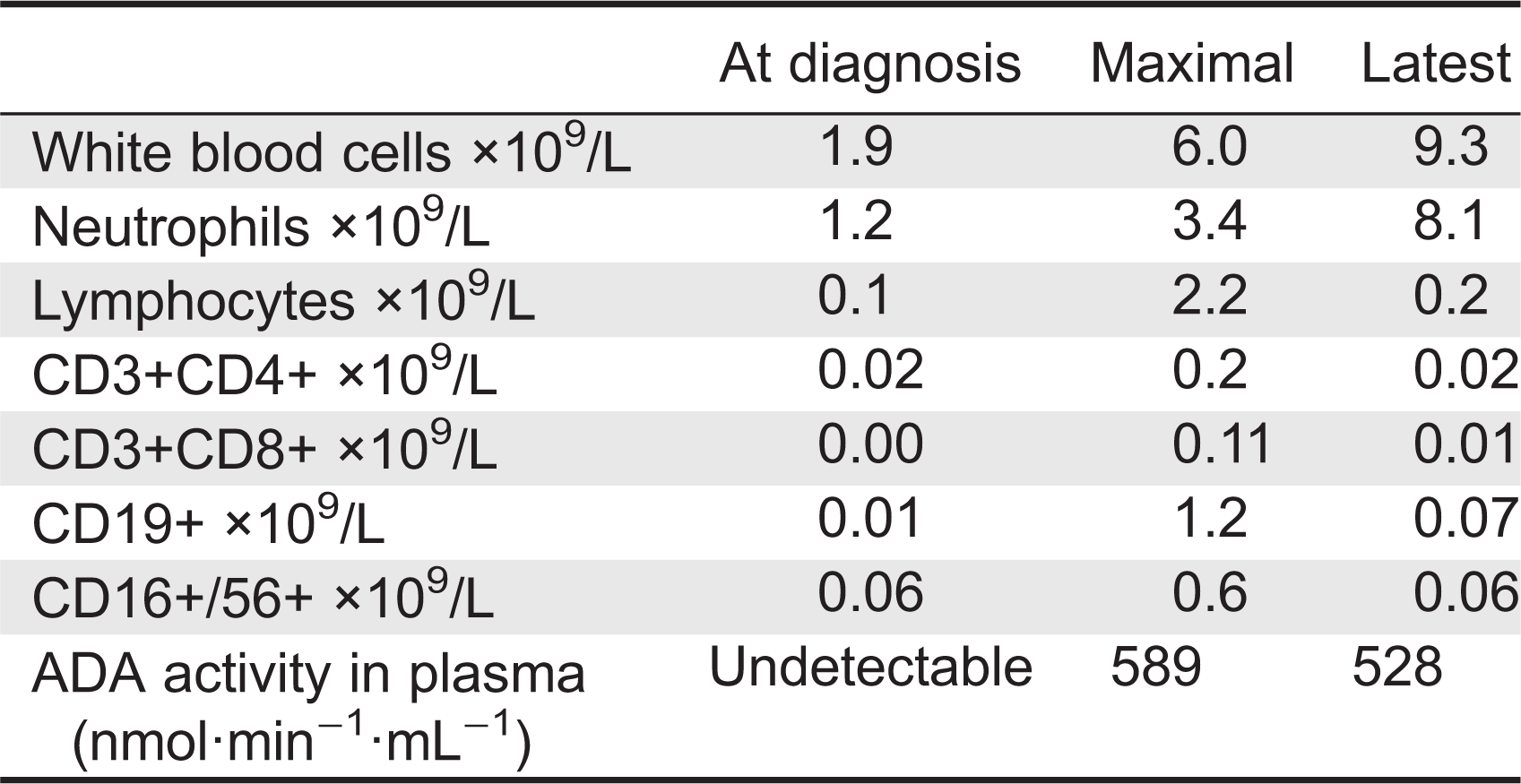
Table 1). The patient also received prophylactic sulfamethoxazole/thrimethoprim and monthly intravenous immunoglobulin (IVIG) infusions without significant infections or autoimmunity. Sensorineural hearing loss was noted in infancy and stage I hypertension, that didn't require treatment, was observed in early childhood. Kidney function and ultrasound were reported to be normal.
At 6 years of age the patient was admitted following a 2-week history of vomiting, fatigue, and increased bruising with normal urine output and no diarrhea. The patient had received IVIG 3 weeks earlier, was reported to have recovered from a mild upper respiratory tract infection, and had normal complete blood count, kidney functions, and urine. Upon admission, the patient was afebrile and normotensive. Initial laboratory investigations revealed markedly elevated creatinine 500 µmol/L (normal 9–55 µmol/L), urea 50 mmol/L (normal 3.0–7.0 mmol/L), and phosphate 3.08 mmol/L (normal 1.30–1.75 mmol/L) as well as low hemoglobin 49 g/L (normal 105–135 g/L) and ionized calcium 0.69 mmol/L (normal 1.10–1.30 mmol/L) with normal coagulation. Blood gas revealed the patient's pH was 7.49 with a base excess of 12.9 mmol/L. Urinalysis showed 2+ blood and protein. All microbiology studies were negative. There was an
E. coli O157:H7 outbreak occurring in the patient's community 2 months prior to her presentation. However there was no known direct contact of the patient with affected individuals nor was Shiga-toxin producing
E. coli isolated from the patient's stools at the time of her acute presentation. All viral cultures (nasopharyngeal and stool) as well as blood cultures remained negative. There was also no history of ingestion of ethylene glycol, methanol, drugs, or other toxins. The patient was treated briefly with ceftriaxone and piperacillin-tazobactam. After 3 days in hospital, she developed nonbloody diarrhea and thrombocytopenia (nadir of 84 × 10
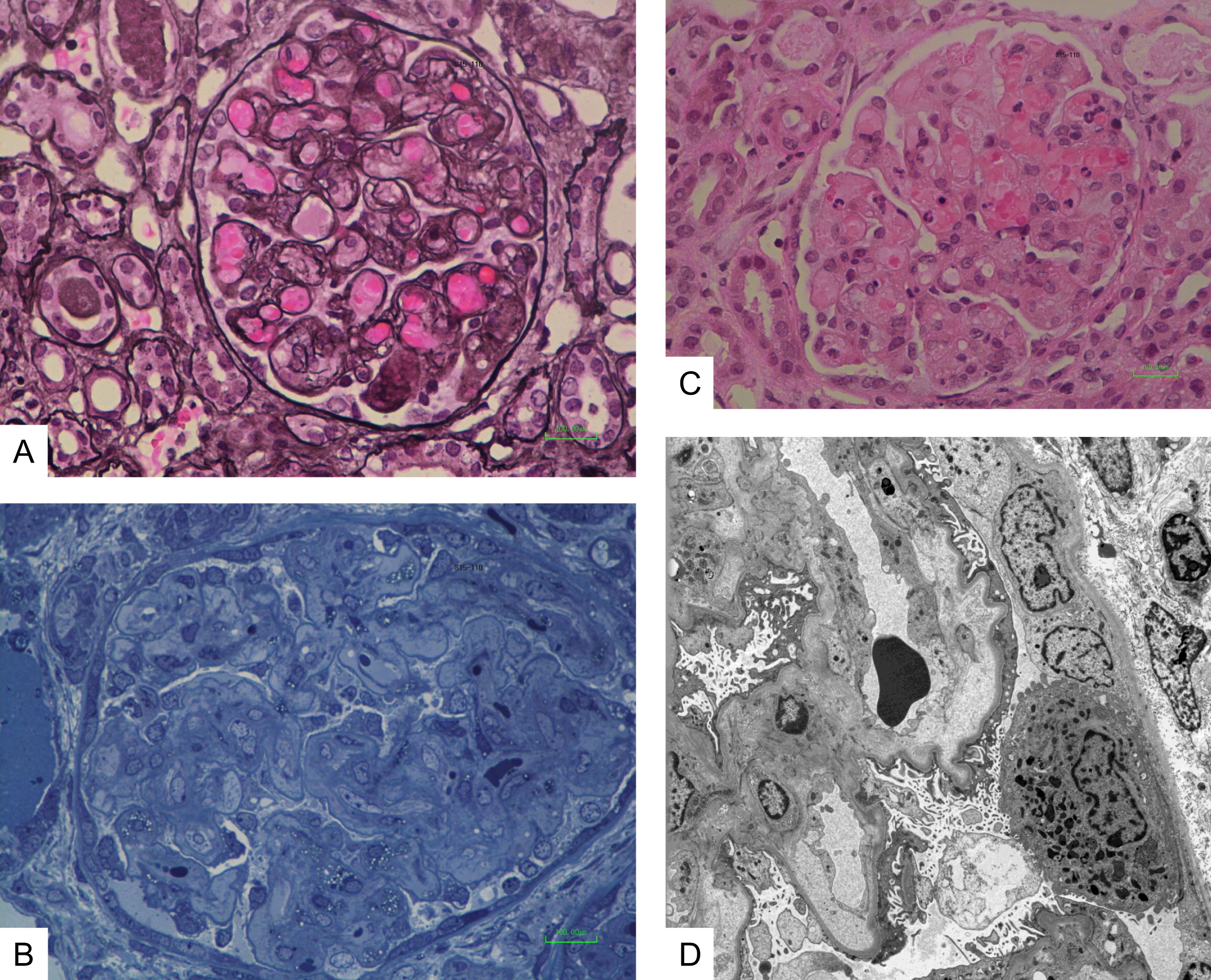
9/L), which resolved after 2 days. A kidney biopsy revealed mesangiolysis and replication of the basement membrane with insudation of plasma into the mesangium and into the glomerular capillary wall (
Figure 1A). There was also thickening of the loops with narrowing and occlusion of the loops by the replicated basement membrane, matrix, and cells (
Figure 1B). Some loops were occluded by erythrocytes and platelet aggregates (
Figure 1C). Electron microscopy confirmed the glomerular mesangiolysis and the accumulation of a pale matrix within the glomerular basement membrane (GBM) with elevation of the endothelial cells and replication of the GBM (
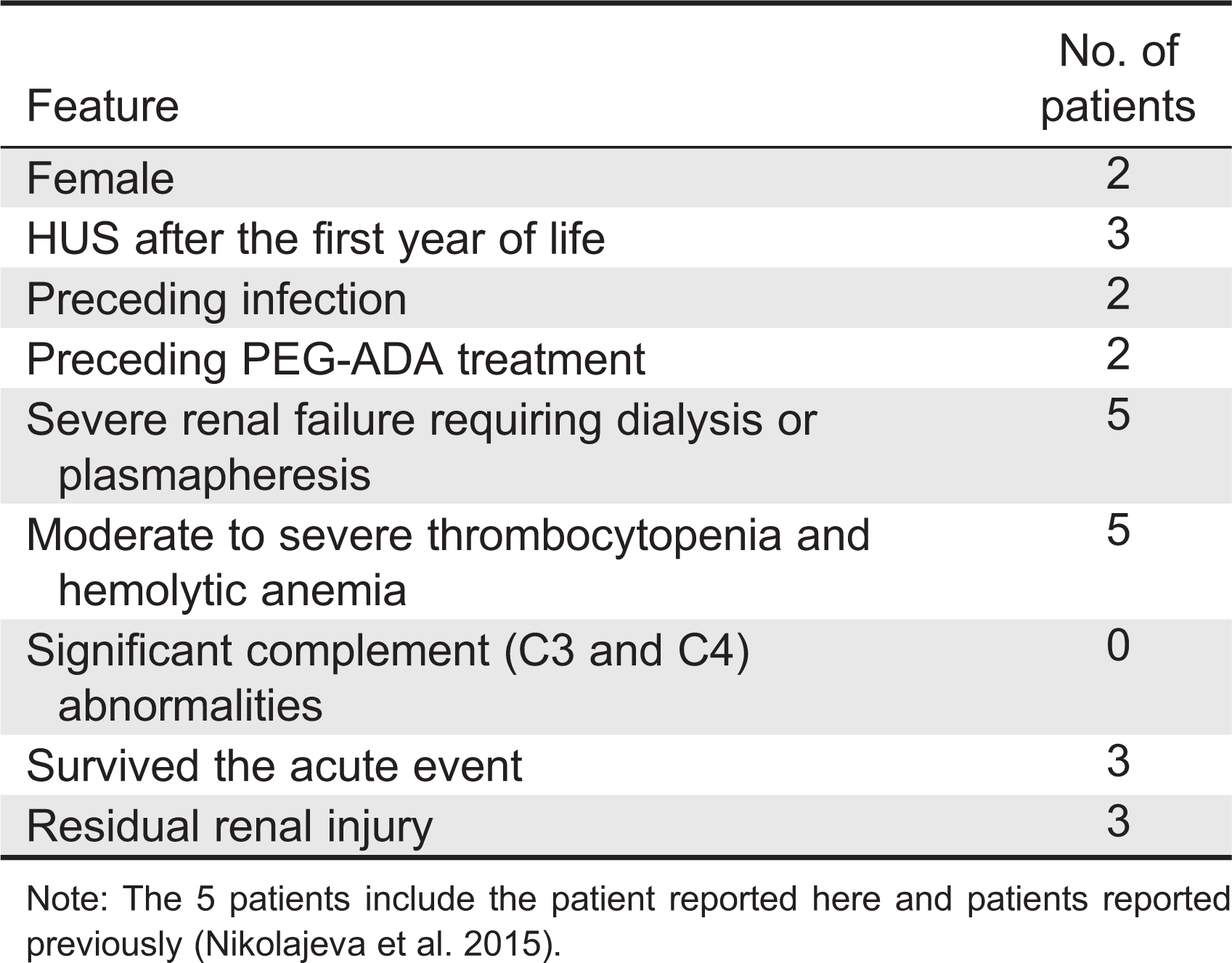
Figure 1D). These features were consistent with a thrombotic microangiopathy. There was no evidence of tubulo-interstitial nephritis. The patient was treated by hemodialysis, with improved kidney function and normalization of the serum creatinine and urea. PEG-ADA and IVIG administration were not interrupted. C3 and C4 levels were within the normal range. ADAMTS13 (von Willebrand factor-cleaving protease) testing was negative. Mutations in genes coding for proteins involved in regulation or activation of the alternative complement activation pathway including factor H, I, membrane cofactor protein, and factor B or C3 were not identified. Antibodies to complement components or Factor H were not detected. Thus, the ADA-deficient patient described here has unequivocal clinical, laboratory, and histological characteristics of HUS with significant kidney disease.