Introduction
Phagocytosis is defined as a receptor-mediated process in which targeted particles are engulfed and degraded. Neutrophils are the predominant leukocytes in peripheral blood and from there, they are mobilized to the sites of infection (
Teng et al. 2017;
Leach et al. 2019). Although neutrophils do not possess the properties for adaptive recognition of antigens, nevertheless, they mediate early innate immune responses to infection and display the capacity to modulate the adaptive immune response (
Ley et al. 2018;
Papayannopoulos 2018;
Silvestre-Roig et al. 2019;
Rosales 2020). Neutrophils are also important sources of pro- and anti-inflammatory cytokines, thus participating in host defenses through a variety of phenotypically simple but mechanistically complex processes (
Tamassia et al. 2018;
Gideon et al. 2019;
Kumar 2020). Other neutrophil functions include chemotaxis, respiratory burst activity, direct bacterial killing, and antibody-dependent cell-mediated cytotoxicity, and most of these functions can be demonstrated in vitro (
Lehman and Segal 2020).
Immunoglobulins are glycoproteins produced by plasma cells and are key effector factors of the adaptive immune system. The Fc regions of IgG molecules are involved in complement and antibody-dependent phagocytosis (
Quast et al. 2017). IgG is the most abundant immunoglobulin and therefore constitutes most of the gamma region in serum protein electrophoresis (
Adedeji et al. 2014). Hypergammaglobulinemia (HGG) is found in many situations. In the setting of primary immunodeficiency, HGG could, for instance, be related to a defect in T cell function, liver diseases, malignancies, autoimmune diseases, and infections (
Upton 2014). Although immunodeficiency may be associated with a significant decrease in plasma IgG, IgM, or IgA isotypes, some immunodeficiency conditions with normal or elevated levels of immunoglobulins have been documented (
Conley et al. 1999;
Adedeji et al. 2014;
Pimenta et al. 2019). Furthermore, neutrophil functions are reduced in the immunodeficiency state, such as in HIV-infection (
Dantas et al. 2015). Some immunodeficiency diseases are very well described as having high levels of a particular immunoglobulin. For instance, hyper IgM usually results from the impaired ability of B cells to undergo immunoglobulin class-switching, while IgE usually predominates in hyper IgE syndrome as well as other conditions (
Lo et al. 2013).
Previous studies have shown that some healthy humans have exhibited HGG (
Buadi et al. 2011;
Adedeji et al. 2015). This phenomenon was first recognized in Nigerians living in Britain as far back as the 1950s (
Schofield 1957), but the precise immunological basis for this condition is still emerging. Since neutrophils are crucial in the first line of defense and immunoglobulins are expressed following antigenic stimulation, we hypothesized that impaired neutrophil phagocytic function would contribute to this phenomenon. Although phagocyte dysfunction has been reported in pathologic conditions (
Carneiro et al. 2012;
Teng et al. 2017), data on phagocytic activity of neutrophils isolated from peripheral blood of apparently healthy individuals exhibiting HGG are scarce. Thus, we investigated whether neutrophil dysfunction is associated with HGG in apparently healthy individuals, as this could provide a possible basis for this phenomenon.
Discussion
In this report, we demonstrated reduced phagocytotic activity in neutrophils isolated from apparently healthy participants exhibiting HGG compared with the controls. We first identified healthy participants showing HGG by SPE, and subsequently confirmed HGG by quantifying the gamma globulin band using GelQuant image analysis and quantitation software (
Khakabimamaghani et al. 2013).
Since HGG has been reported in patients with chronic liver disease (
Fallatah and Akbar 2010), we ruled out this condition by studying the liver and renal indices such as plasma AST, ALT, ALP, urea and creatinine. The liver and renal indices and HIV status reports confirmed that HGG is not due to underlying liver disease in the study participants. Other demographic parameters in both HGG and control participants were essentially the same (
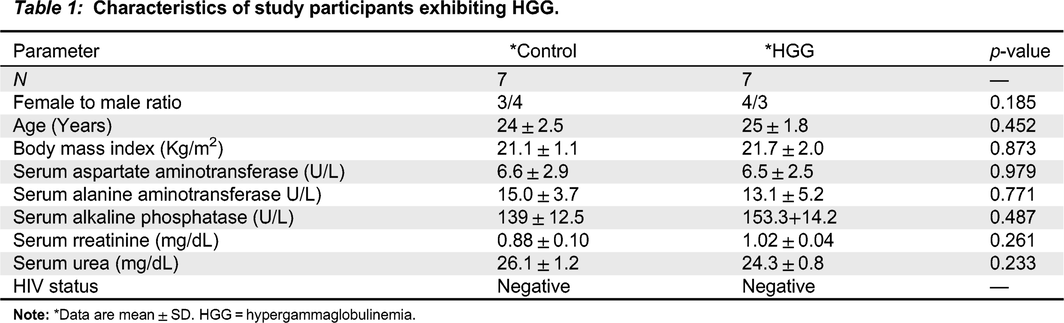
p > 0.05) (
Table 1).
SPE is invaluable in health and diseases. It has 3 traditional applications; the first application is in the identification and characterization of serum protein abnormalities, the second application is in identifying the transition from monoclonal gammopathy of undetermined significance to multiple myeloma, and the third application is in disease activity or treatment in multiple myeloma (
Katzmann et al. 1997). The procedure is now very useful in identifying healthy humans exhibiting HGG. Different varieties of SPE have been reported depending on the nature of the support medium employed. The 3 commonly used support media, cellulose acetate, agarose, and capillary, each have different specificity and sensitivity in determining serum protein abnormalities (
Katzmann et al. 1997). It is noteworthy that the cellulose acetate support medium used in this study has been reported to have excellent specificity.
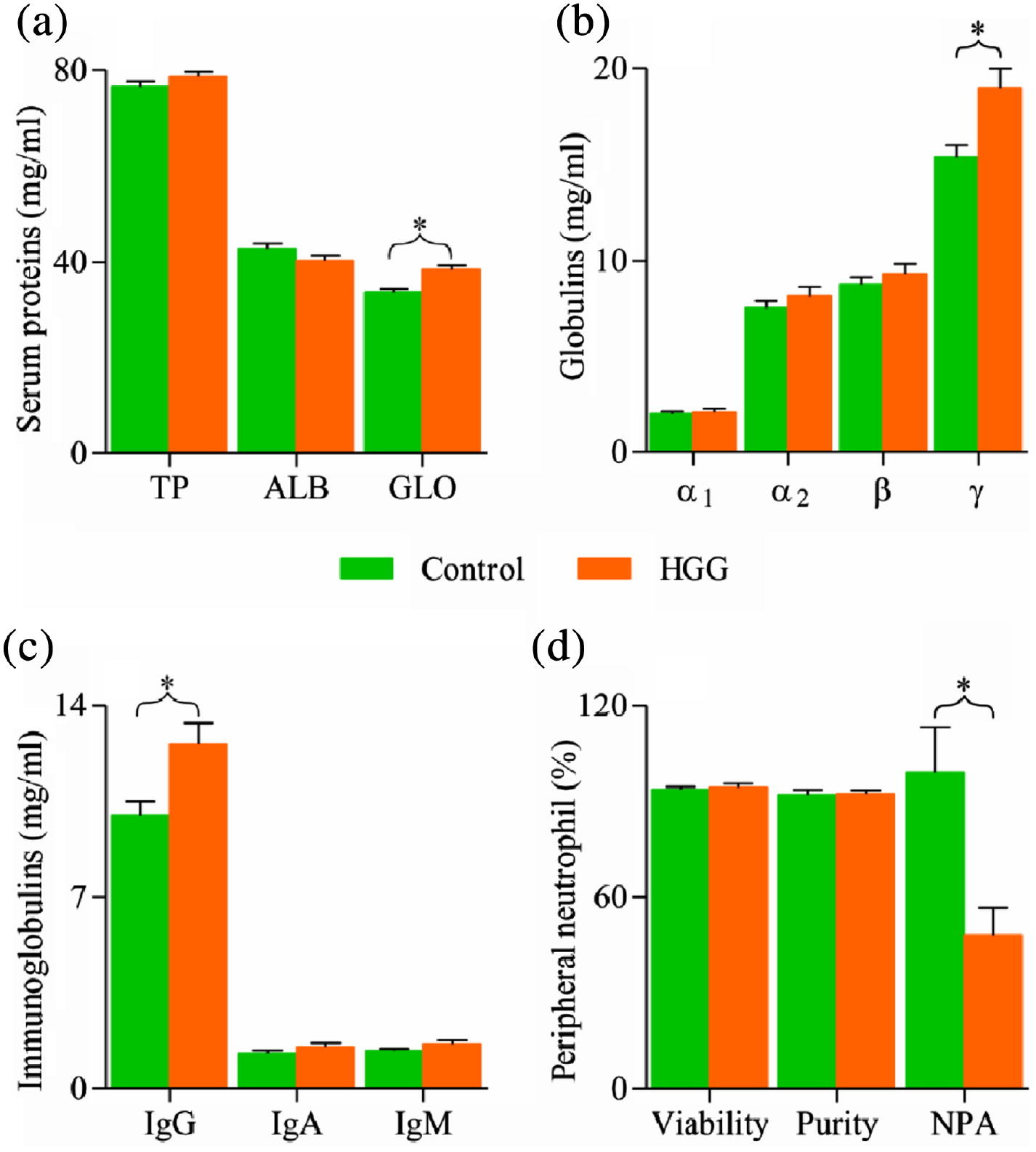
The concentration of serum gamma globulin and IgG were significantly (
p < 0.05) higher in the HGG group compared to the control. Immunofixation electrophoresis (IFE) is a research tool for identifying the electrophoretic mobility of serum protein fractions in blood and other biological fluids. With the use of IFE, several proteins have been identified to migrate in the gamma band of SPE. Notably amongst them is IgG. Others are IgA, IgM, and complement C3 (
Yousif et al. 2018). In this study, gamma globulin and total IgG were positively correlated and it is evident from previous reports that the major component of the gamma band is IgG. Phagocytic activity of neutrophils was demonstrated to be significantly reduced in participants exhibiting HGG. Anomalies in neutrophil phagocytic function have been observed in several common medical and surgical conditions (
Engelich et al. 2001). Its occurrence among participants exhibiting HGG is still emerging.
As is generally known, phagocytosis is initiated by the interaction of phagocytic receptors with ligands on the surface of target microbes. Then, receptors aggregate to initiate signaling pathways that regulate the actin cytoskeleton, so that the phagocyte can produce membrane protrusions to engulf microbes. Lastly, the microbes are encircled in a new vesicle that protrudes out from the plasma membrane. The impairment in NPA may result from 1 or more of the many well-defined and complex processes that have been systematically divided into 4 principal steps: recognition of pathogens, activation of the internalization process, formation of the phagosome, and phagolysosome maturation. These processes have been recently reviewed (
Nordenfelt and Tapper 2011;
Rosales and Uribe-Querol 2017;
Liew and Kubes 2019). Further studies will be needed to demonstrate precisely the molecules that are defective in this condition. However, phagocytic defects are generally a consequence of impaired actin polymerization around phagosomes (
May and Machesky 2001;
Baranov et al. 2016).
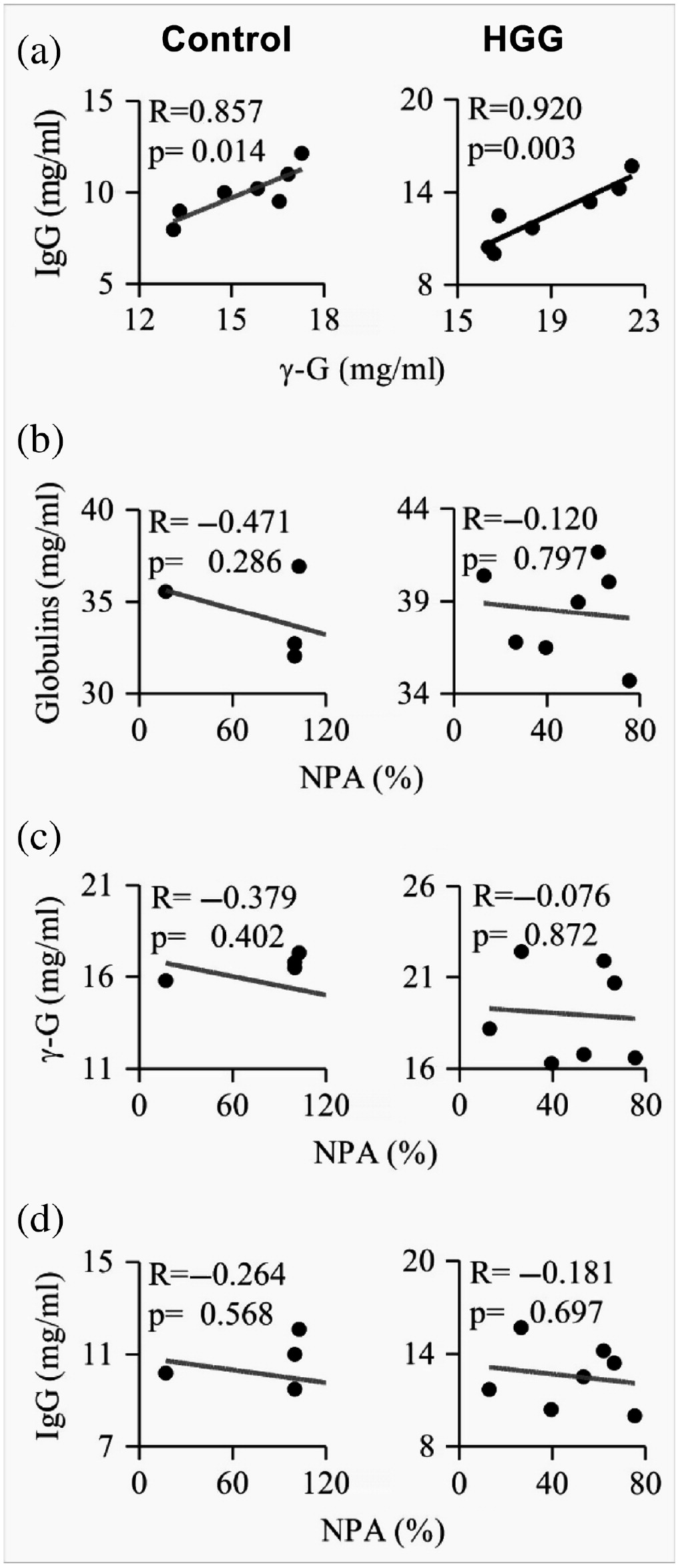
The correlation studies show that IgG is implicated in HGG and significant correlation was observed between total IgG and gamma globulins (
Figure 1), and these may be expressed against microbes that breached the first line of defense. Determination of specific antibodies would add an interesting dimension to this study. Furthermore, evaluation of the IgG subtype may reveal a predominant subclass in this condition. For instance, IgG1 and IgG3 subclass predominate in HIV infection and liver cirrhosis, respectively (
Riggione et al. 1983;
Kekow et al. 1988).
Since neutrophils play crucial roles as effector molecules in the first line of defense in humans, impairment of neutrophil phagocytic activity may favour persistent antigenic stimulation of the adaptive immune system. This in turn could orchestrate gamma globulin expression leading to HGG. Consequently, there might be a reduced ability of neutrophils, in this subset of young individuals, to combat microbial infections. However, since no peculiar symptom is associated with reduced neutrophil phagocytosis in apparently healthy individuals considered in this study, this phenomenon could be termed reduced neutrophil phagocytic activity of undetermined significance.