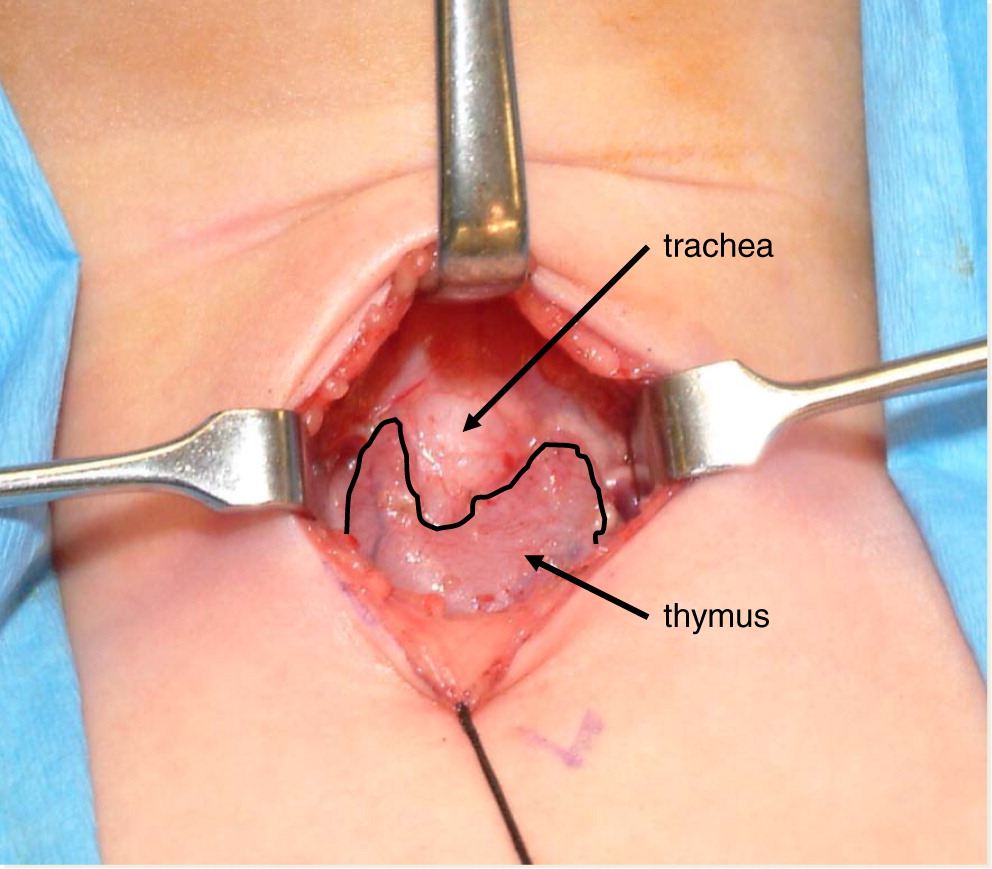
The thymus gland was found to be normal in 5 patients (
Table 2). In 2 of these 5 patients (p10, p12), the biopsy results helped to avoid bone marrow transplantation (BMT). In the other 3 patients, the pathology findings were useful in further defining the diagnosis and identifying novel immunodeficiencies.
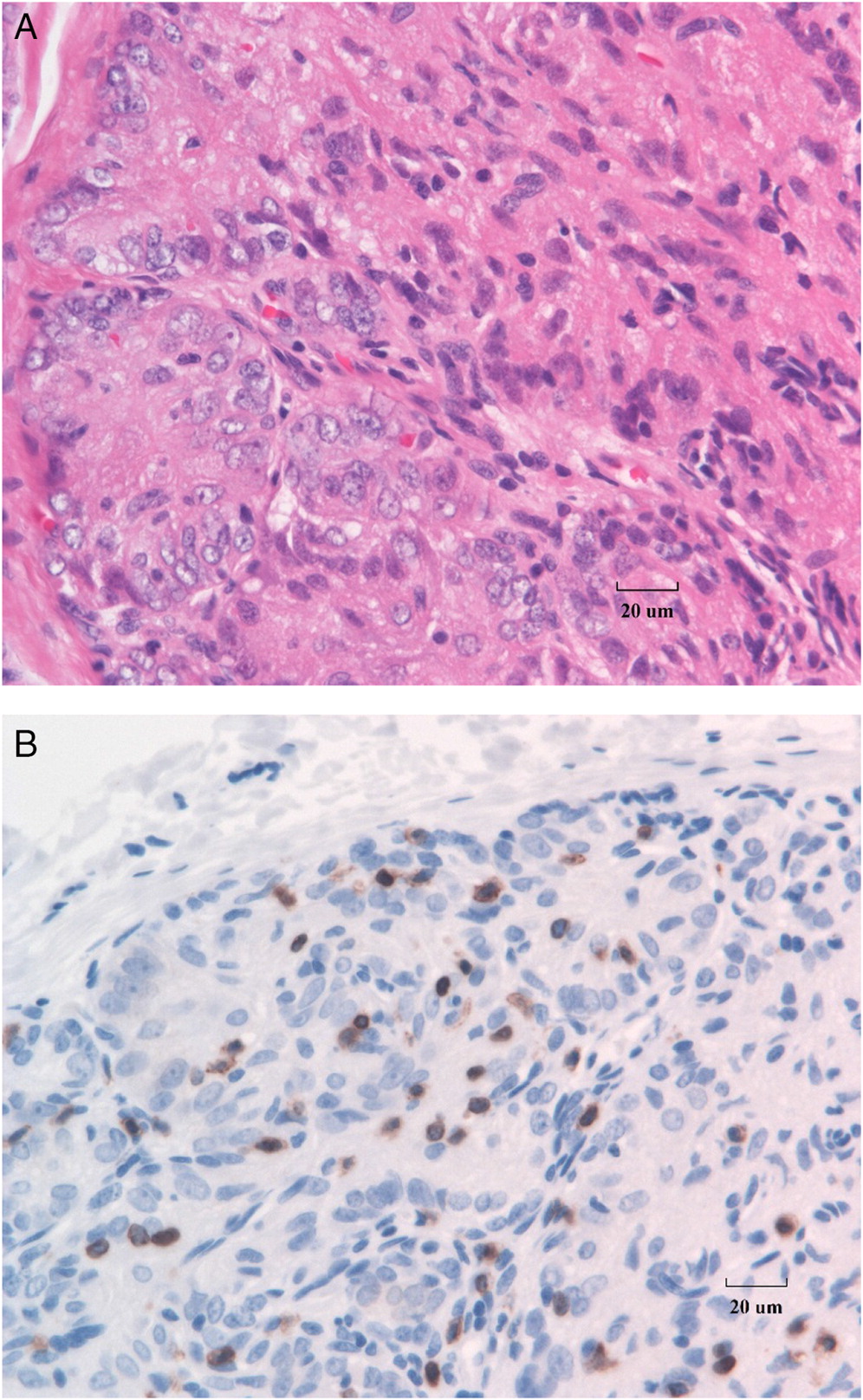
The rest of the patients had morphological abnormalities of the thymus gland. Classic dysplasia of the thymus in SCID, with loss of architecture, depletion of lymphocytes, loss of cortico-medullary differentiation, and absence of Hassall’s corpuscles is shown in
Figure 2A. Immunostaining for T cells with anti-CD3 typically demonstrates marked absence of T cells in the thymus (
Figure 2B). Further, detection of cytokeratin in the SCID thymus reveals CK18 expression in the medullary spindle cell component of the thymic epithelial cells as well as cortical surface staining (
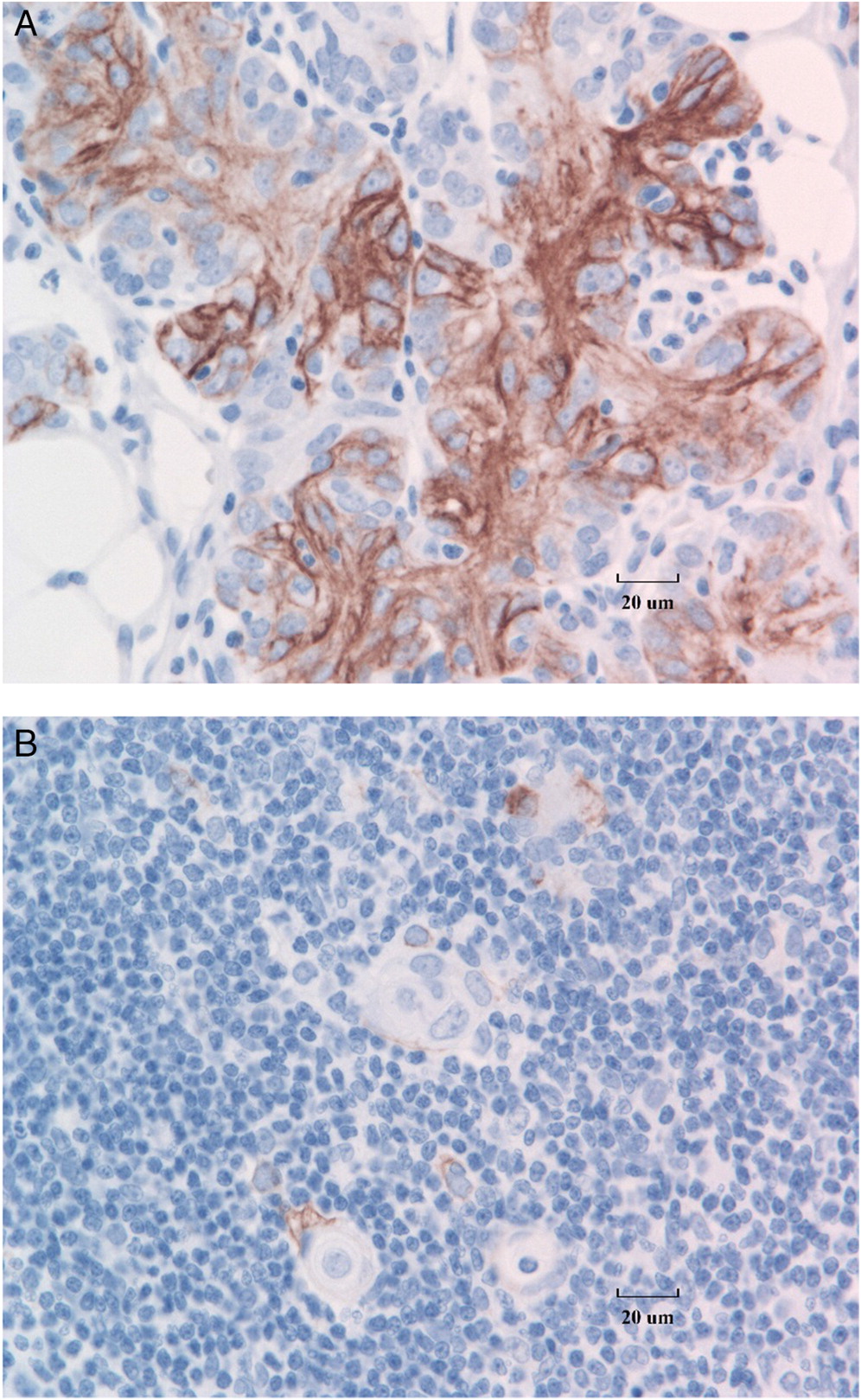
Figure 3A), as compared with CK18 staining of normal thymic tissue control (
Figure 3B). Immunostaining with a cocktail of antibodies for CK8 and CK18 shows strong expression in both cortical polygonal thymic epithelial cells and spindle cells (
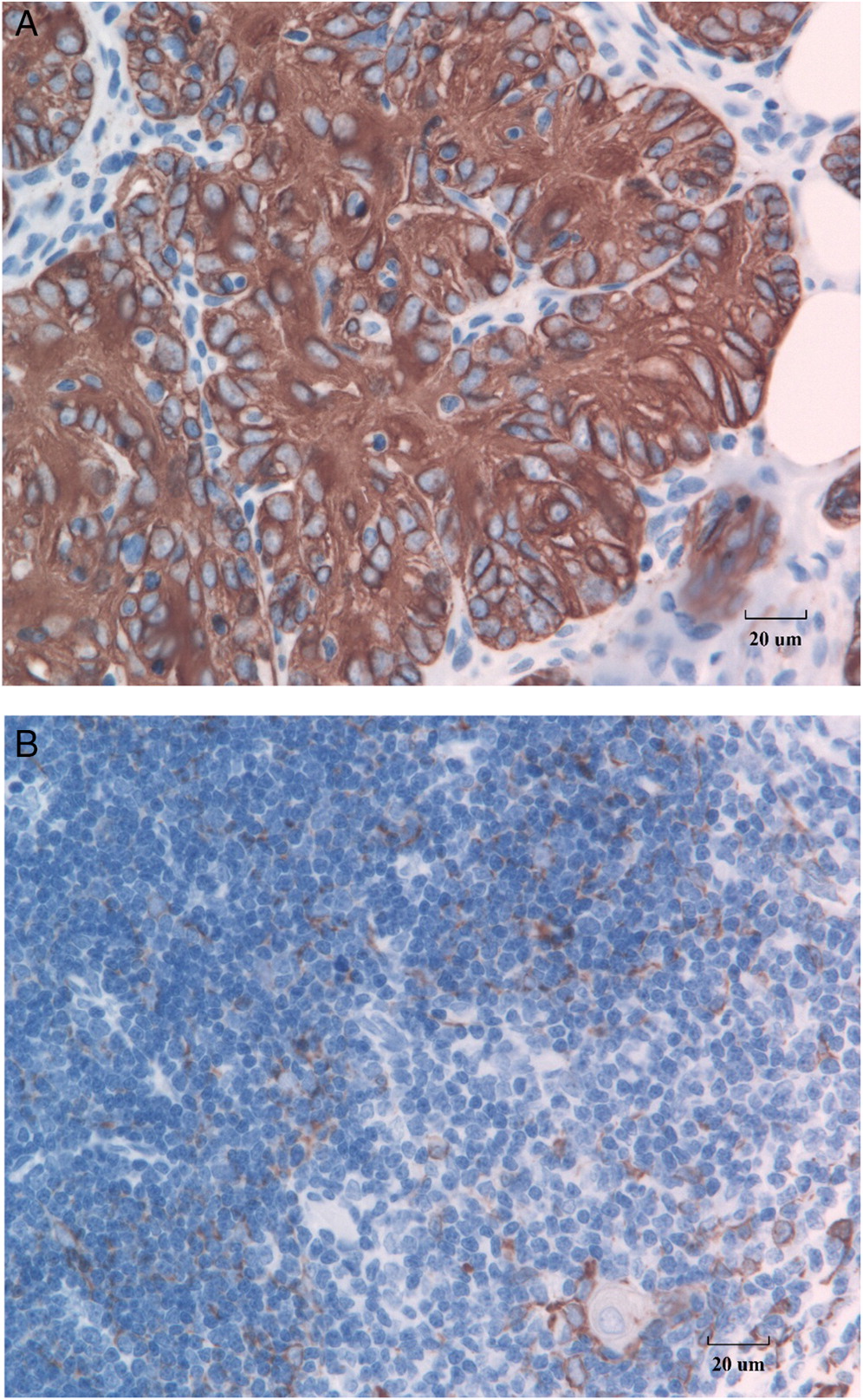
Figure 4A) as compared with the staining pattern in a normal thymus (
Figure 4B). In contrast to the healthy control thymus, in which cortical thymic epithelial cells stain negatively for CK5 and CK6 and medullary thymic epithelial cells stain positively for these markers (
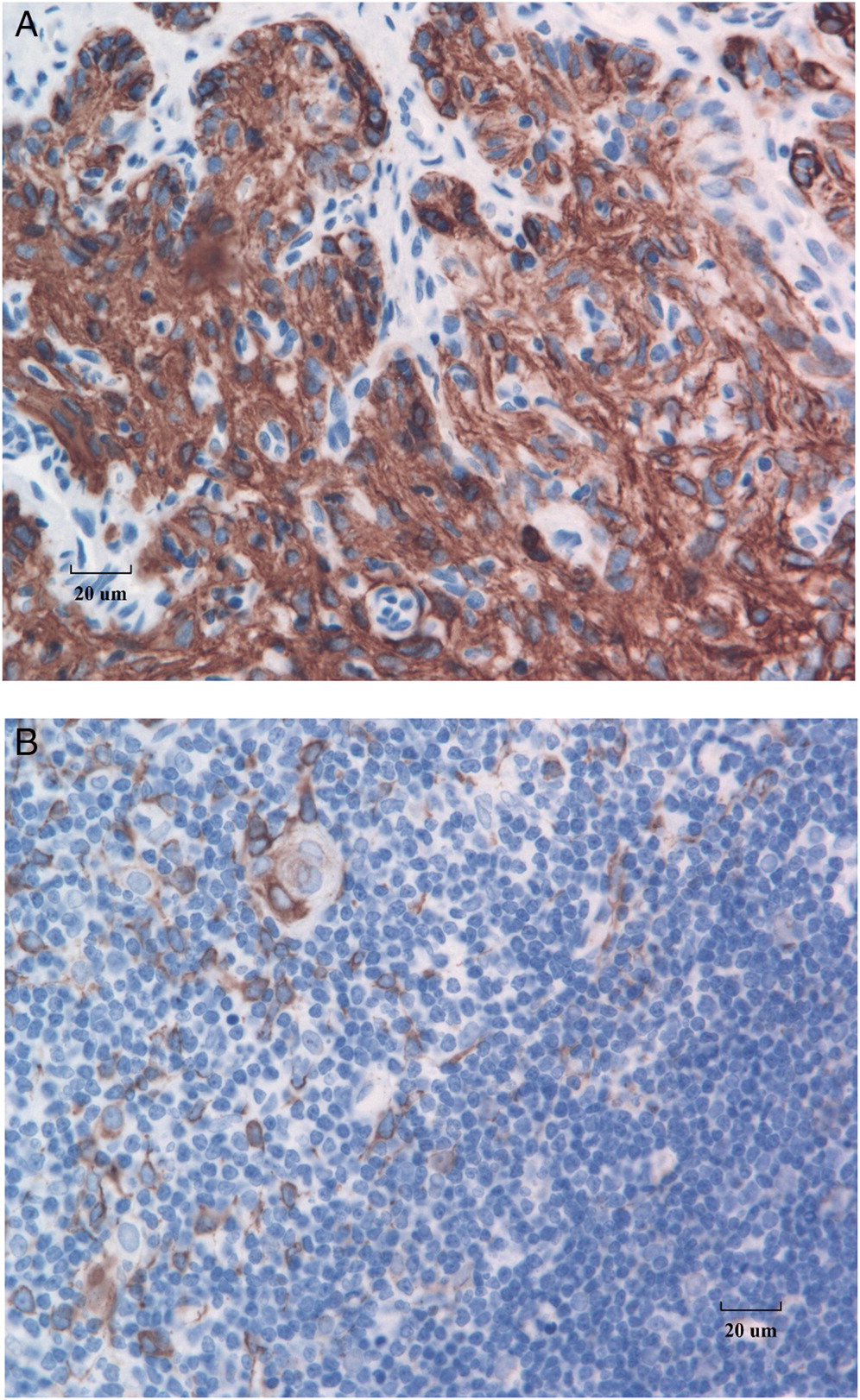
Figure 5B), in SCID, these cells all strongly express CK5 and CK6 (
Figure 5A).
Three patients had all the hallmarks of a dysplastic thymus including loss, or near loss of lobular structure and cortico-medullary distinction, replacement of thymocytes with epithelioid cells and lack of Hassall’s corpuscles. In these patients the results supported the decision to perform BMT. The other 6 patients had various degrees of thymus gland abnormalities. In 5, Hassall’s corpuscles were either absent or barely developed suggestive of a profound T cell defect. Indeed, all 5 were offered BMT. One of these patients also had bone marrow failure (p3) which was by itself an indication for BMT. In 1 patient (p3) the thymus biopsy was critical in defining the final diagnosis of a novel type of SCID, CD3δ deficiency (
Dadi et al. 2003), in another (p4) changes were mild to moderate and did not warrant further intervention.