Our abstract describes the cases of 3 siblings who arrived as refugees to Canada in 2017. The parents are first cousins and there was an extensive history of consanguinity among relatives. They were determined to have a new homozygous splice site mutation resulting in LRBA deficiency. The oldest sibling presented with a unique manifestation which has yet to be reported in the literature, lymphocytic infiltration of the bladder.
Parents reported that she was well until the age of 3 when she began to have recurrent sinopulmonary infections, including an estimated 10 episodes of otitis media per year. She had arthritis involving her left knee since age 4 which had been treated with prednisone. Trials of methotrexate for 2 years and adalimumab for 1 year had been unsuccessful. At 4 years old she developed severe diarrhea with 6–7 watery bowel movements per day, which was ongoing at the time of her initial assessment. She also had severe failure to thrive, with a weight at 50th % for a 4.5 year old and significantly delayed bone age.
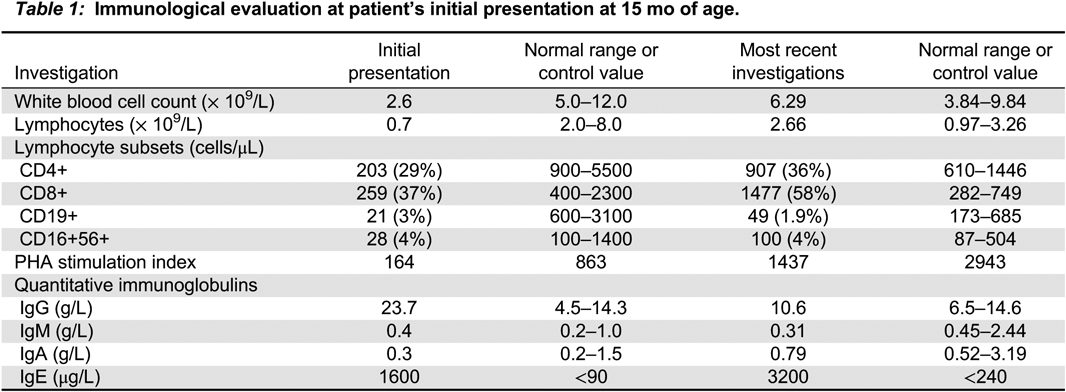
Investigations revealed low immunoglobulins with IgG <0.5 g/L, IgM 0.25 g/L, and undetectable IgA (<0.1 g/L) in the context of normal albumin. On flow cytometry she had low B-cells at 4% (just below the 5th % for her age), with 1% class-switched memory B-cells. CD4+ and CD8+ T cells were appropriately represented. NK cells were low at 2%, which is below the 5th % for her age. Specific antibody titres were not measured as the patient’s vaccination history was unclear. Lymphocyte proliferation to mitogens was normal.
Due to these findings, along with her severe symptoms, she was started on replacement immunoglobulin at 1 g/kg. Despite this, infections remained problematic. In February 2018, she developed increasing watery stools which were positive for Campylobacter. Initial treatment with oral antibiotics was unsuccessful and the patient developed Clostridium difficile. She was admitted and treated with 2 courses of IV meropenem. Unfortunately her diarrhea resumed when meropenem was discontinued. Endoscopy demonstrated chronic autoimmune/reactive inflammation in her bowel, as well as Candida esophagitis. A biopsy of the gastric antrum was positive for HHV6. The candida was successfully treated with caspofungin. In consultation with the Infectious Disease team, the HHV6 was not treated. Next Generation Sequencing of 247 primary immunodeficiency genes revealed a homozygous splice site variant mutation in LRBA at the locus c.2258+2T>G, confirming the patient’s diagnosis of LRBA deficiency.
Due to ongoing GI symptoms in the context of LRBA deficiency and previously reported success of Abatacept in this patient population, biweekly Abatacept infusion (20 mg/kg) was initiated. There was no obvious initial improvement after 6 weeks, therefore Sirolimus (1.3 mg BID) was added, and TPN was started. The diarrhea improved significantly on this regimen, as well as a maintenance dose of oral steroid. After 1 month, TPN was weaned and weight was maintained with oral supplementation. Infectious prophylaxis with daily cephalexin was added to her monthly IVIG.
Additional characteristics of LRBA deficiency in this patient included chronic CMV viremia and recurrent HSV-1 with oral ulcers. The CMV was treated with Ganciclovir but she developed ITP and neutropenia, making it unclear whether this were secondary to underlying disease or the medication. Her initial hepatomegaly improved significantly after the CMV was treated. She had significant shortness of breath, exercise intolerance, and orthopnea. A CT scan revealed multi-lobar cystic bronchiectasis. She was also noted to have hypokalemia with renal loss of both protein and potassium. This was thought to be secondary to an underlying tubulopathy, although biopsy was not performed. An echocardiogram showed a large aorta, the etiology which has not been established.
Extensive infectious investigations of urine, blood, and stool failed to isolate a pathogen. Urinalysis showed microscopic hematuria as well as 1 g/L of proteinuria. This was confirmed with a repeat urinalysis a week later. A kidney biopsy was performed which revealed thin basement membrane disease and sclerotic glomeruli. Urology was involved and initial urodynamic studies were normal. Cystoscopy showed bladder and urethral irritation. Biopsies revealed primarily T-lymphocytic infiltration, but also eosinophils, neutrophils and rare plasma cells. The biopsy was also positive for EBV but blood remained EBV negative. The ultimate pathological diagnosis was interstitial cystitis.
Immunological investigations were initiated and showed low immunoglobulins with IgG 6.29 g/L, IgM 0.08 g/L, and IgA <0.1 g/L. Albumin was normal. On flow cytometry B-cells were 1% (below the 5th %), with 3% class-switched memory B-cells. CD4+, CD8+ T cells, and NK cell numbers were normal. Titres were positive for Rubella, Measles and Hepatitis B but negative for Tetanus, Diphtheria, and Pneumococcus. However, these results were challenging to interpret given the unclear vaccination history. DNA sequencing revealed the same homozygous mutation in the LRBA gene as his sister.
Trials of Hydroxyzine and Sirolimus were both unsuccessful in reducing symptoms, which progressed to include urinary frequency. Prednisone 1 mg/kg/day and gabapentin 100 mg BID were initiated. This treatment regimen helped to lessen his symptoms significantly. He was also started on monthly IVIG.
The patient continues to have difficulty with urinary frequency, urgency, and dysuria. He has now developed pelvic floor dysynergy and has rectal pain as well. This is being managed with physiotherapy and anti-spasmodic agents. He also developed enteropathy with watery stools at age 13, and was initially treated for Campylobacter. Despite resolution of the infection, enteropathy recurred and is currently managed with Abatacept. Growth is being supported with NG feeds.
REFERENCES
Alangari, A., Alsultan, A., Adly, N., Massaad, M.J., Kiani, I.S., Aljebreen, A., Raddaoui, E., Almomen, A.K., Al-Muhsen, S., Geha, R.S., and Alkuraya, F.S. 2012. LPS-responsive beige-like anchor (LRBA) gene mutation in a family with inflammatory bowel disease and combined immunodeficiency. J. Allergy Clin. Immunol. 130(2):481–488.e2. PMID: 22721650. doi: 10.1016/j.jaci.2012.05.043.
Alkhairy, O.K., Abolhassani, H., Rezaei, N., Fang, M., Andersen, K.K., Chavoshzadeh, Z., Mohammadzadeh, I., El-Rajab, M.A., Massaad, M., Chou, J., Aghamohammadi, A., Geha, R.S., and Hammarström, L. 2016. Spectrum of phenotypes associated with mutations in LRBA. J. Clin. Immunol. 36(1):33–45. PMID: 26707784. doi: 10.1007/s10875-015-0224-7.
Azizi, G., Abolhassani, H., Mahdaviani, S.A., Chavoshzadeh, Z., Eshghi, P., Yazdani, R., Kiaee, F., Shaghaghi, M., Mohammadi, J., Rezaei, N., Hammarström, L., and Aghamohammadi, A. 2017. Clinical, immunologic, molecular analyses and outcomes of Iranian patients with LRBA deficiency: A longitudinal study. Pediatr. Allergy Immunol. 28(5):478–484. PMID: 28512785. doi: 10.1111/pai.12735.
Charbonnier, L.M., Janssen, E., Chou, J., Ohsumi, T.K., Keles, S., Hsu, J.T., Massaad, M.J., Garcia-Lloret, M., Hanna-Wakim, R., Dbaibo, G., Alangari, A.A., Alsultan, A., Al-Zahrani, D., Geha, R.S., and Chatila, T.A. 2015. Regulatory T-cell deficiency and immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like disorder caused by loss-of-function mutations in LRBA. J. Allergy Clin. Immunol. 135:217–227. PMID: 25468195. doi: 10.1016/j.jaci.2014.10.019.
De Bruyne, M., Bogaert, D.J., Venken, K., Van den Bossche, L., Bonroy, C., Roels, L., Tavernier, S.J., van de Vijver, E., Driessen, A., van Gijn, M., Gámez-Diaz, L., Elewaut, D., Grimbacher, B., Haerynck, F., Moes, N., and Dullaers, M. 2018. A novel LPS-responsive beige-like anchor protein (LRBA) mutation presents with normal cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and overactive TH17 immunity. J. Allergy Clin. Immunol. 142(6):1968–1971. PMID: 30193839. doi: 10.1016/j.jaci.2018.08.026.
Eren Akarcan, S., Edeer Karaca, N., Aksu, G., Aykut, A., Yilmaz Karapinar, D., Cetin, F., Aydinok, Y., Azarsiz, E., Gambineri, E., Cogulu, O., Ulusoy Severcan, E., Alper, H., and Kutukculer, N. 2018. Two male siblings with a novel LRBA mutation presenting with different findings of IPEX syndrome. JMM Case Rep. 5(10):e005167. PMID: 30479781. doi: 10.1099/jmmcr.0.005167.
Gámez-Díaz, L., August, D., Stepensky, P., Revel-Vilk, S., Seidel, M.G., Noriko, M., Morio, T., Worth, A.J.J., Blessing, J., Van de Veerdonk, F., Feuchtinger, T., Kanariou, M., Schmitt-Graeff, A., Jung, S., Seneviratne, S., Burns, S., Belohradsky, B.H., Rezaei, N., Bakhtiar, S., Speckmann, C., Jordan, M., and Grimbacher, B. 2016. The extended phenotype of LPS-responsive beige-like anchor protein (LRBA) deficiency. J. Allergy Clin. Immunol. 137(1):223–230. PMID: 26768763. doi: 10.1016/j.jaci.2015.09.025.
Kostel Bal, S., Haskologlu, S., Serwas, N.K., Islamoglu, C., Aytekin, C., Kendirli, T., Kuloglu, Z., Yavuz, G., Dalgic, B., Siklar, Z., Kansu, A., Ensari, A., Boztug, K., Dogu, F., and Ikinciogullari, A. 2017. Multiple presentations of LRBA deficiency: A single-center experience. J. Clin. Immunol. 37(8):790–800. PMID: 28956255. doi: 10.1007/s10875-017-0446-y.
Lévy, E., Stolzenberg, M.C., Bruneau, J., Breton, S., Neven, B., Sauvion, S., Zarhrate, M., Nitschké, P., Fischer, A., Magérus-Chatinet, A., Quartier, P., and Rieux-Laucat, F. 2016. LRBA deficiency with autoimmunity and early onset chronic erosive polyarthritis. Clin. Immunol. 168:88–93. PMID: 27057999. doi: 10.1016/j.clim.2016.03.006.
Lo, B., Zhang, K., Lu, W., Zheng, L., Zhang, Q., Kanellopoulou, C., Zhang, Y., Liu, Z., Fritz, J.M., Marsh, R., Husami, A., Kissell, D., Nortman, S., Chaturvedi, V., Haines, H., Young, L.R., Mo, J., Filipovich, A.H., Bleesing, J.J., Mustillo, P., Stephens, M., Rueda, C.M., Chougnet, C.A., Hoebe, K., McElwee, J., Hughes, J.D., Karakoc-Aydiner, E., Matthews, H.F., Price, S., Su, H.C., Rao, V.K., Lenardo, M.J., and Jordan, M.B. 2015. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science. 349(6246):436–440. PMID: 26206937. doi: 10.1126/science.aaa1663.
Lo, B., Fritz, J.M., Su, H.C., Uzel, G., Jordan, M.B., and Lenardo, M.J. 2016. CHAI and LATAIE: New genetic diseases of CTLA-4 checkpoint insufficiency. Blood. 128:1037–1042. PMID: 27418640. doi: 10.1182/blood-2016-04-712612.
Lopez-Herrera, G., Tampella, G., Pan-Hammarström, Q., Herholz, P., Trujillo-Vargas, C.M., Phadwal, K., Simon, A.K., Moutschen, M., Etzioni, A., Mory, A., Srugo, I., Melamed, D., Hultenby, K., Liu, C., Baronio, M., Vitali, M., Philippet, P., Dideberg, V., Aghamohammadi, A., Rezaei, N., Enright, V., Du, L., Salzer, U., Eibel, H., Pfeifer, D., Veelken, H., Stauss, H., Lougaris, V., Plebani, A., Gertz, E.M., Schäffer, A.A., Hammarström, L., and Grimbacher, B. 2012. Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. Am. J. Hum. Genet. 90(6):986–1001. PMID: 22608502. doi: 10.1016/j.ajhg.2012.04.015.
Martínez Jaramillo, C., and Trujillo-Vargas, C.M. 2018. LRBA in the endomembrane system. Colomb. Med. 49(3):236–243. PMID: 30410199.
Serwas, N.K., Kansu, A., Santos-Valente, E., Kuloğlu, Z., Demir, A., Yaman, A., Gamez Diaz, L.Y., Artan, R., Sayar, E., Ensari, A., Grimbacher, B., and Boztug, K. 2015. Atypical manifestation of LRBA deficiency with predominant IBD-like phenotype. Inflamm. Bowel Dis. 21:40–47. PMID: 25479458. doi: 10.1097/MIB.0000000000000266.
Soler-Palacín, P., Garcia-Prat, M., Martín-Nalda, A., Franco-Jarava, C., Rivière, J.G., Plaja, A., Bezdan, D., Bosio, M., Martínez-Gallo, M., Ossowski, S., and Colobran, R. 2018. LRBA deficiency in a patient with a novel homozygous mutation due to chromosome 4 segmental uniparental isodisomy. Front. Immunol. 9:2397. PMID: 30386343. doi: 10.3389/fimmu.2018.02397.
Wang, J.W., Gamsby, J.J., Highfill, S.L., Mora, L.B., Bloom, G.C., Yeatman, T.J., Pan, T.C., Ramne, A.L., Chodosh, L.A., Cress, W.D., Chen, J., and Kerr, W.G. 2004. Deregulated expression of LRBA facilitates cancer cell growth. Oncogene. 23(23):4089–4097. PMID: 15064745. doi: 10.1038/sj.onc.1207567.