Introduction
Chronic granulomatous disease (CGD) is a rare primary immunodeficiency disease (PID) of impaired reactive oxygen species (ROS) production caused by inherited defects in the Nicotinamide Adenine Dinucleotide Phosphate reduced (NADPH)-Oxidase enzyme complex. The NADPH oxidase complex generates reactive oxygen species (ROS), which are required for intracellular pathogen killing through pH changes and potassium influx (
Reeves et al. 2002). Furthermore, defects in NADPH activity and lack of ROS formation also impair extracellular pathogen killing by neutrophil extracellular traps (
Romao et al. 2015). Ineffective ROS generation in patients with CGD leads to recurrent infections and hyper-inflammation. Mutations in any of the 5 components of the NADPH complex cause CGD. Mutations in the
CYBB gene encoding the gp91
phox protein causes X-linked CGD, whereas bi-allelic mutations in
CYBA,
NCF1,
NCF2, and
NCF4 genes encoding p22
phox, p47
phox, p67
phox, and p40
phox proteins, respectively, cause autosomal recessive (AR) CGD (reviewed in
Leiding and Holland 2012).
In patients with typical clinical features compatible with CGD, the diagnosis of CGD can be reached by demonstrating lack of ROS production in neutrophils. However, establishing an unequivocal diagnosis of CGD requires genetic testing. Reaching a genetic diagnosis is clinically important since most X-linked CGD patients have a more severe phenotype and a worse prognosis compared with p47
phox-deficient CGD; therefore, offering a curative HSCT is based not only on the clinical phenotype of the patient but also on the genetic cause of CGD (
Leiding and Holland 2012). The somewhat milder phenotype in AR CGD compared to X-linked CGD, as manifested by increased survival, is associated with residual NADPH activity mostly in P47
phox-deficient AR CGD (
Kuhns et al. 2010).
CGD is a rare disease with a prevalence ranging from as high as 1:79 000 (
Leiva et al. 2007) to 1:450 000 (
Ǻhlin et al. 1995), but the prevalence and incidence are higher in the consanguineous populations in Israel. The latest report shows a birth incidence of 1.05 per 100 000 live births in the Jewish population versus 1.45 per 100 000 live births in the more consanguineous Arab Israeli population (
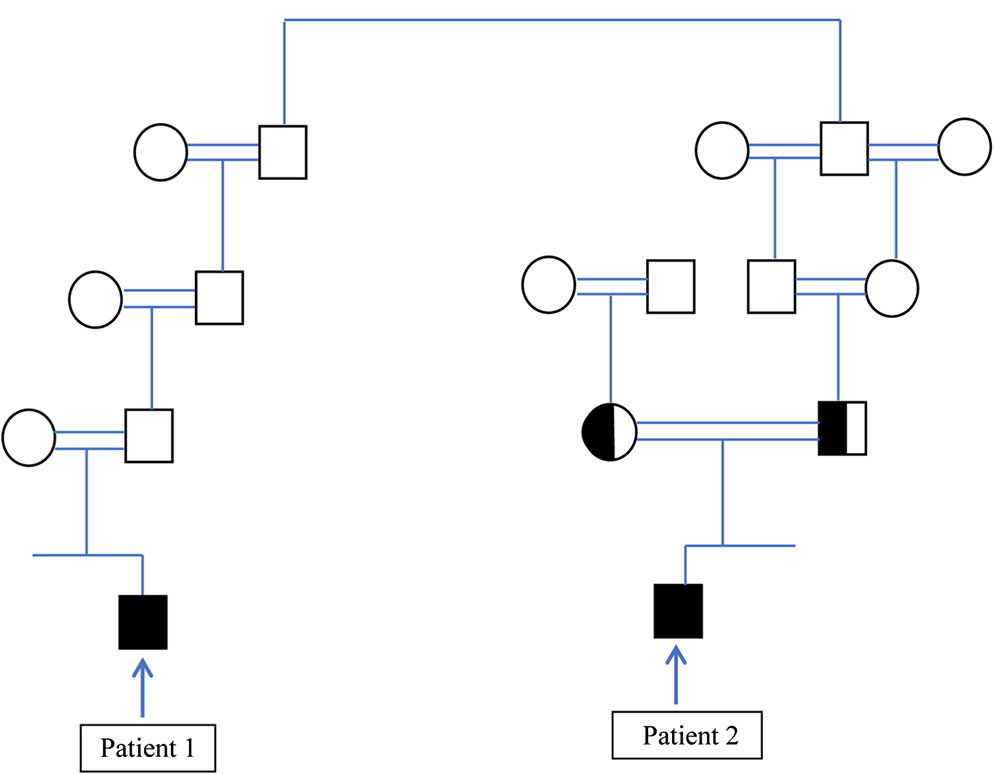
Wolach et al. 2017). Therefore, the assumption that the diagnosis of 2 children with CGD in 1 consanguineous, extended family is caused by the same genetic mutation is reasonable. Nevertheless, we report on the diagnosis of 2 children, from the same extended family, with typical CGD, who eventually were diagnosed as having different genetic causes of CGD.
The genetic studies reported here were performed following an informed consent.
Discussion
In this report we show that CGD can occur in the same extended family due to different genetic causes. It seems reasonable to assume that patients from the same family with a distinct PID phenotype will have the same genetic cause of disease, and if 1 patient in the family is diagnosed with a disease-causing mutation, then other patients from the same family will have the same mutation, particularly in highly consanguineous families. In this report we show that this assumption may be erroneous. In many developing countries genetic testing for rare genetic diseases is not readily available, and the costs of genetic testing may be charged to the family and not performed for this reason. In such instances, genetic testing may be performed only for 1 patient and not for other family members, even though they may have the same (as yet undetected) disease. Indeed, after the diagnosis of patient 1, 2 additional brothers were diagnosed as having CGD even though they did not have typical infections. If we would have assumed that the mutation discovered in patient 2 is the same disease-causing mutation in patient 1, we would have missed the correct genetic diagnosis.
Although different rare genetic primary immunodeficiency diseases may co-exist in the same family (
Broides et al. 2009) and sometimes even in the same patient (
Ehlayel et al. 2008), the present report adds to our knowledge by showing that the same primary immunodeficiency, in this case CGD, can be due to different genetic causes even within 1 extended family.
It is noteworthy that patient 2 had some residual NADPH activity compared to patient 1 who had virtually no NADPH activity, and indeed patient 2 displayed a milder phenotype compared with patient 1. These different clinical courses can be expected based on the residual NADPH activity in patient 2 (
Kuhns et al. 2010).
Identifying the genetic defect is essential and crucial. It will aid in offering prenatal diagnosis, early diagnosis and treatment to affected children and may have therapeutic implications. Such is the case in CGD, since patients with X-linked CGD who have a severe phenotype, and no residual NADPH activity may be offered a HSCT more readily than those with AR CGD who have a milder phenotype and some residual NADPH activity. Indeed, the clinical course of patient 1 with X-linked CGD was more severe than patient 2 with AR CGD.
This report shows the importance of reaching the correct genetic diagnosis in PID patients, even when performing such studies may seem redundant due to the assumption that patients with a similar phenotype from an extended consanguineous family should have the same genetic cause of their disease.