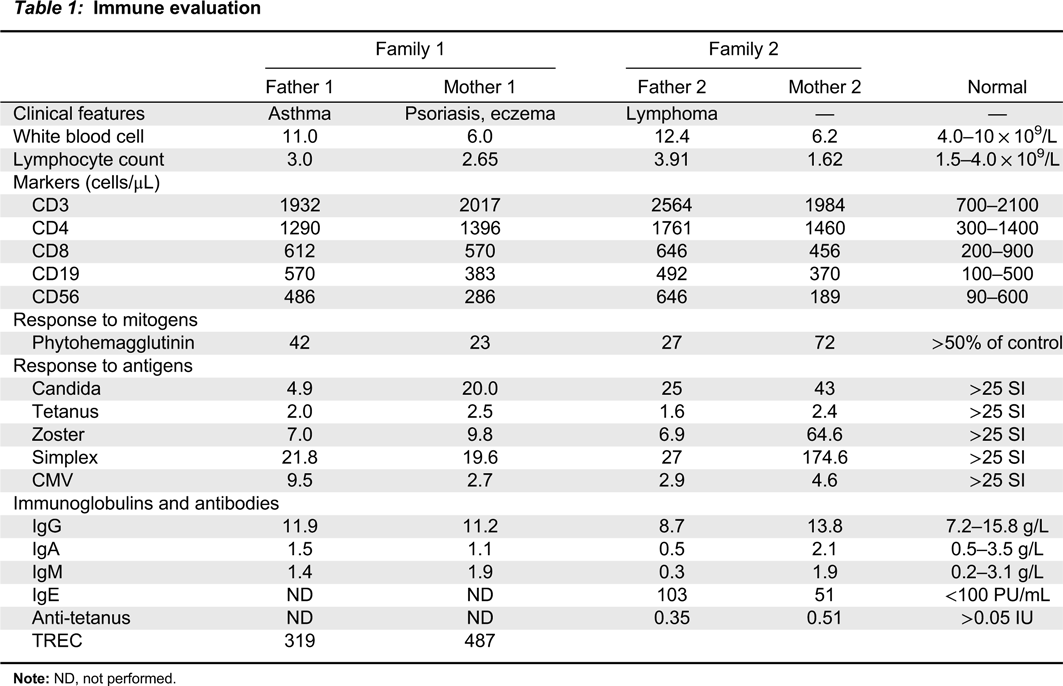
A novel type of combined immunodeficiency associated with a homozygous mutation in the
RelB gene has recently been described (
Merico et al. 2015;
Sharfe et al. 2015). Here we describe the clinical and immunologic features of 4 patients, the parents of the homozygous
RelB patients, all whom were discovered to have heterozygous
RelB mutations. The 3 patients described with homozygous
RelB mutations were identified as having a premature stop codon mutation leading to markedly decreased levels of RelB mRNA, presumably due to message instability (
Merico et al. 2015). Furthermore, RelB protein was undetectable in these patient’s lymphocytes, suggesting that any potential fragment that may be translated is also unstable and subsequently degraded (
Merico et al. 2015).
Sharfe et al. (2015) detailed the effects of
RelB deficiency on lymphocyte development and function (
Sharfe et al. 2015). In particular, patients with homozygous
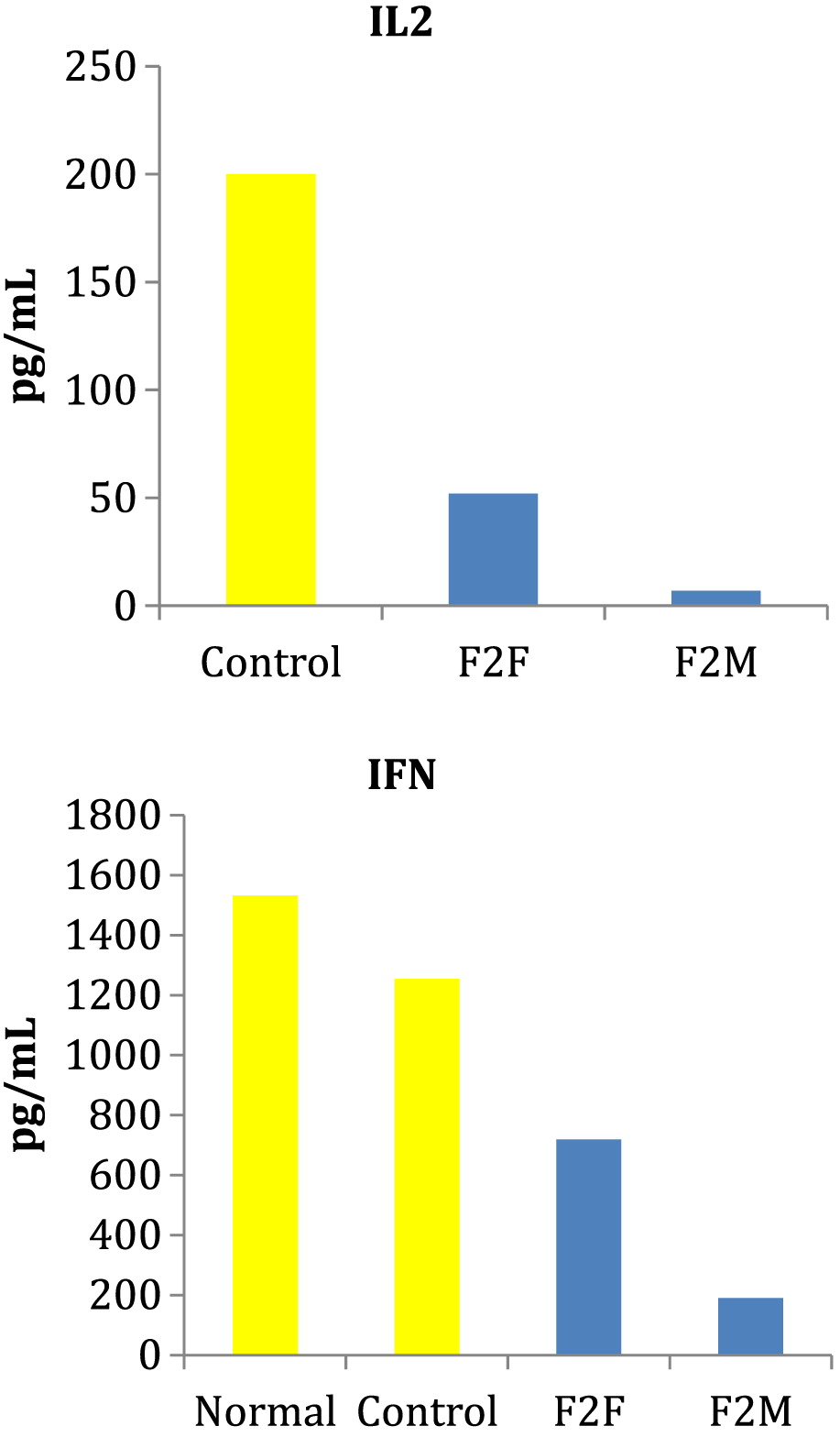
RelB mutations have T-cell dysfunction as evident by markedly dysplastic thymus with reduced production of T cells, abnormal TCR repertoire with clonal expansion, depressed in vitro responses to T-cell mitogens and antigens, reduced production of IL2 and IFNγ, and exaggerated in vitro responses to anti-CD3 and CD28 (
Sharfe et al. 2015). Furthermore, B-cell dysfunction was demonstrated with reduced BAFF receptor expression, absent memory/mature B cells and low to absent T-cell dependent antibody responses (
Sharfe et al. 2015). The result of the intrinsic defects in both T- and B-lymphocyte maturation and function was a presentation consistent with combined immunodeficiency with features including repeated infections and failure to thrive. The 4 patients described here with heterozygous
RelB mutations did not have recurrent infections as a clinical feature but did have evidence of immune dysregulation. Specifically, our patients also had impaired T-cell function with inadequate response to mitogens and antigens as well as reduced IL2 and IFNγ production following stimulation with PHA. These findings suggest that heterozygous
RelB mutations also result in deficient T lymphocytes in vivo. The lack of recurrent infections in our patients may reflect residual RelB function and (or) the involvement of compensatory mechanisms protective against infection in the group with heterozygous
RelB mutations.
Impaired immune functioning is also evident by the lymphoma that developed in F2. Aberrant NFκB activity has been observed in many cancers, including both solid and hematopoietic malignancies (
Basseres and Baldwin 2006;
Naugler and Karin 2008). Evidence suggests that NFκB pathways may mediate pro-apoptotic effect, growth arrest, and inhibition of cancer development (
Perkins 2004;
Chen and Castranova 2007). Specifically, a recent study demonstrated that RelB provides cell proliferation-inhibitory signals in murine fibroblasts and that RelB ectopic expression inhibits xenograft tumor growth in vivo, whereas RelB knockdown enhances it;
Jacque et al. (2013) proposed these findings were secondary to direct transcriptional activation of the p53 tumor-suppressor gene by RelB. These findings highlight the importance of malignancy surveillance in this patient population.