Introduction
Hereditary angioedema (HAE) was first described by William Osler in 1888. However, it was not until 1963 that Donaldson and Evan first described the biochemical abnormality responsible for HAE, the absence of C1 esterase inhibitor (C1-INH) in patients with the disease (
Cicardi et al. 2014).
Traditionally, the literature has described two types of HAE, Type I and Type II. Both are autosomal-dominant disorders resulting from mutation in the C1-INH gene on chromosome 11. More than 200 mutations in the gene have been described (
Kaplan 2010). There has been no correlation found between genotype and clinical phenotype in Type I and Type II HAE (
Caccia et al. 2014).
Type I HAE accounts for approximately 80%–85% of all cases. The mutations in Type I HAE can occur anywhere in the gene. Abnormal proteins that are either misfolded or truncated are inefficiently secreted. This results in decreased antigenic levels and functional activity of the C1-INH protein (
Zuraw 2008).
Type II HAE mutations, about 15%–20% of all HAE cases, usually involve single amino acid substitutions, mainly at exon 8 at or near the active site. Levels of circulating C1-INH are usually normal. The secreted protein is dysfunctional, resulting in normal antigenic C1-INH levels but low function C1-INH (
Zuraw 2008).
In addition to Type I and II, another type of hereditary angioedema is recognized. It has been called HAE with normal C1-INH, Type III HAE, or estrogen-dependent hereditary angioedema. Hereditary angioedema with normal C1-INH manifests with sporadic recurrent angioedema but normal C1-INH level and function. The diagnosis of HAE with normal C1-INH is made with meeting the following criteria: (
i) a history of recurrent angioedema in the absence of concomitant hives or concomitant use of a medication known to cause angioedema; (
ii) documented normal or near normal C4, C1-INH level, and C1-INH function; and (
iii) demonstration of a Factor XII mutation that is associated with the disease or a positive family history of angioedema and documented evidence of lack of efficacy of chronic high dose antihistamine therapy (cetirizine at 40 mg/day or the equivalent, for at least 1 month and an interval expected to be associated with 3 or more attacks of angioedema) (
Zuraw et al. 2012). In some cases, bradykinin accumulation appears dependent on Factor XII. The majority of patients do not have any well-described mutations; a small percentage of patients have mutations in Factor XII. This type of angioedema appears to predominantly affect females in high estrogen states. The postulated mechanism by which estrogen exacerbates angioedema is by increasing multiple components of the kallikrein–kinin system (
Duan et al. 2009;
Riedl 2013;
Levy et al. 2014).
Acquired angioedema can also be secondary to a malignancy (usually lymphoproliferative disorders) and autoimmune diseases and present with identical clinical and lab profiles as HAE.
Although not discussed in this review of hereditary angioedema, it is important to ask about medications including angiotensin converting enzyme inhibitor and nonsteroidal anti-inflammatory drug use in patients presenting clinically with angioedema without urticaria.
The prevalence of Type I and II HAE is uncertain but is estimated to be approximately 1 case per 50,000 persons, without known differences among ethnic groups (
Zuraw 2008).
Pathophysiology
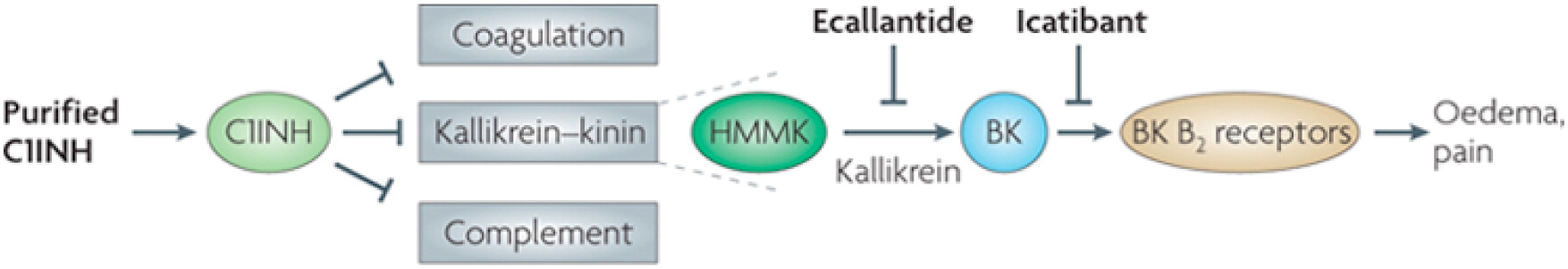
In Type I HAE and Type II HAE, there is a deficiency of C1-INH, a protein serine protease inhibitor that is involved in the complement, coagulation, and contact pathways (
Longhurst and Cicardi 2012). It has been shown that an overproduction of bradykinin, a nonapeptide, in the contact pathway is the main mediator of the increased vascular permeability in Type I and Type II HAE (
Cugno et al. 2009). The deficiency in C1-INH decreases the normal inhibition of kallikrein (
Longhurst and Cicardi 2012), which enables greater cleavage of high molecular-mass kininogen by kallikrein and a resulting increased generation of bradykinin (
Cugno et al. 2009). Abnormally high plasma levels of bradykinin can increase swelling by binding to its cognate receptors (the bradykinin B2 receptor) on vascular endothelial cells, thereby increasing tissue permeability, vascular dilation, and vascular smooth muscle relaxation (
Cugno et al. 2009).
Figure 1 shows a simplified diagram of C1-INH.
Diagnosis
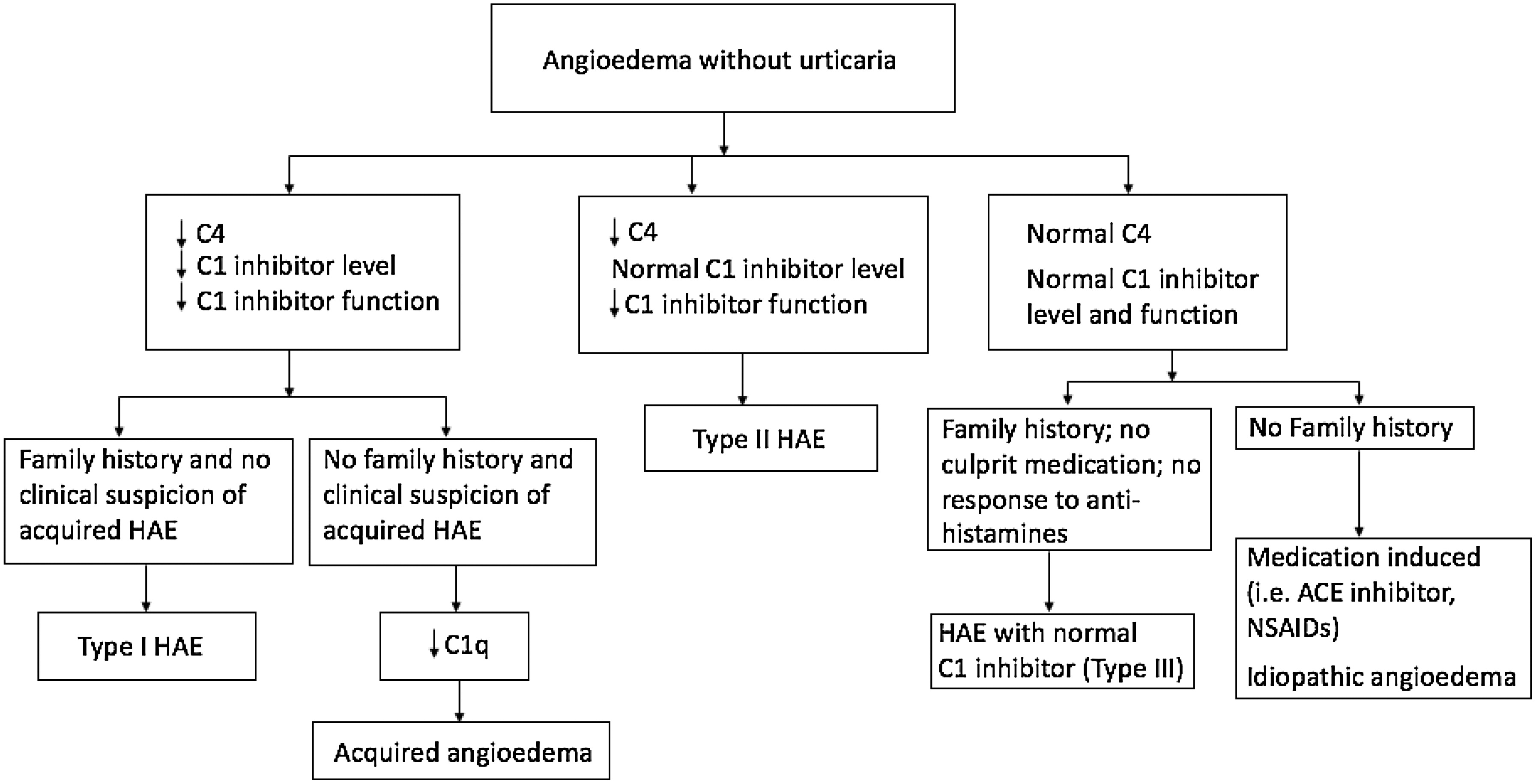
Plasma C4 levels are a valuable screening test for Type I and Type II HAE, with most of those affected having a reduced level between attacks (
Zuraw et al. 1986) and nearly 100% having a low level during attacks (
Lang et al. 2012). If C4 is low, further tests can distinguish Type I HAE patients who have low antigenic C1-INH levels and low functional C1-INH levels from Type II patients with normal antigenic C1-INH levels but low functional C1-INH levels. C1q and C3 are normal with Type I and Type II HAE patients (
Zuraw 2008). Reduction in C4 and C1-INH levels and function of C1-INH should be confirmed one to three months after initial testing (
Cicardi et al. 2014).
Figure 3 shows a diagnostic algorithm.
Two methods (chromogenic or immunoenzymatic) are available to measure C1-INH function. They are based on the measurement of the capacity of plasma to inhibit the esterase activity of a fixed amount of C1s. The chromogenic assay is usually preferred owing to a higher positive predictive value of close to 100%. It is important to send the C1-INH function to specialized laboratories to avoid inaccurate results. The samples should be stored at –20°C (
Wagenaar-Bos et al. 2008). Genetic testing is not necessary for the diagnosis given the heterogeneity of mutations responsible for Type I and Type II HAE (
Cicardi et al. 2014). Genetic testing is also not widely available. The average time between the onset of symptoms and diagnosis was 22 years as of 1977 and was still more than 10 years as of 2005 (
Zuraw 2008).
The importance of diagnosis was shown in a study by
Bork et al. (2012). Mortality by asphyxiation due to laryngeal angioedema was significantly higher in patients with undiagnosed HAE (63 cases) than in patients diagnosed with HAE (7 cases). HAE patients must be informed of the risks of laryngeal angioedema and be educated about emergency treatments and the need for early treatment administration (
Bork et al. 2012).