Chronic Granulomatous Disease (CGD) is a rare, inherited defect of leukocyte phagocytic function with an inability to produce superoxide radicals. It is the result of a mutation in at least 1 of 5 genes that encode nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex enzyme. Two of the protein subunits are membrane-bound glycoprotein gp91
phox (phagocyte oxidase) and p22
phox; the remaining subunits are cytosolic p47
phox, p67
phox, and p40
phox (
Segal et al. 2000).
The prevalence of CGD differs by country with estimated prevalence up to 1:111 000 to 1:1 330 000 in Israeli Arabs and the United Kingdom to as low as 1:1 000 000 in a recent Italian cohort (
Jones et al. 2008;
Martire et al. 2008;
Wolach et al. 2008). In North America, the reported disease prevalence is 1:200 000–250 000 with 70% of patients having the mutation in the X-linked
CYBB gene encoding gp91
phox. Other gene mutations are autosomal recessive (AR); the most common AR gene affected is neutrophil cytosolic factor 1 (
NCF1), encoding p47
phox (
Winkelstein et al. 2000). In the Middle East, most patients have autosomal recessive mutations as opposed to the X-linked
CYBB gene (
Fattahi et al. 2011;
Al-Muhsen and Alsum 2012;
Koker et al. 2013).
Functional and clinical presentation
A 12-year-old Pakistani male of consanguineous parents presented with migratory arthritis and painless oral ulcerations of 6 months duration. The arthritis involved metacarpopharyngeal (MCP), metatarsopharyngeal (MTP), and proximal interphalangeal (PIP) joints at various time points. However, within a week, the patient had diffuse arthritis in most MCP, MTP, and PIP joints of the hands and feet. He had accompanying arthralgias in his neck, shoulder, elbows, wrists, and knees. Painless oral ulcerations were swabbed with negative results for viruses. The arthritis resolved within a week with a nonsteroidal anti-inflammatory (ibuprofen) and without the use of antimicrobial drugs.
Four months later, his arthritis relapsed and he developed daily fevers, anorexia, fatigue, and weight loss. Blood work showed anemia (Hgb 110 g/L), platelets (172 × 109/L), white blood cells (5.2 × 109/L), and differential (neutrophils 2.5 × 109/L, lymphocytes 2.2 × 109/L). Inflammatory markers were elevated (ESR 62, CRP 29). Rheumatological work-up included a weakly positive antinuclear antibody (ANA) (titer 1:40), negative staining for anti-DsDNA, anti-RNP, anti-Smith, anti-cardiolipin, anti-SS-A, anti-SS-B, and rheumatoid factor. He had transient response to naproxen. His fever persisted and he was found to have angular stomatitis, cervical lymphoadenopathy, hepatomegaly, and arthritis in the left PIP joints of the hand and in the right ankle. Repeated blood work was significant for a normocytic anemia (Hgb 95 g/L), thrombocytopenia (Plt 141 × 109/L), elevated LDH 1603 U/L, ferritin 1230 mcg/L, ESR 127, CRP 9.3, hypertiglycerdemia (3.2 mmoL/L), and mild transaminitis (ALT 63, AST 87). Bone marrow aspirate was not suggestive of hemophagocytosis. Mildly elevated soluble CD 136 (1086 ng/mL) and soluble IL-2 receptor (CD25) (1698 U/mL) were noted. The patient was treated for presumptive Macrophage Activation Syndrome given his arthritis history and blood work. He received 60 mg of oral prednisone with complete symptom resolution, and he was discharged on a tapering schedule. Again, no antimicrobial agents were used.
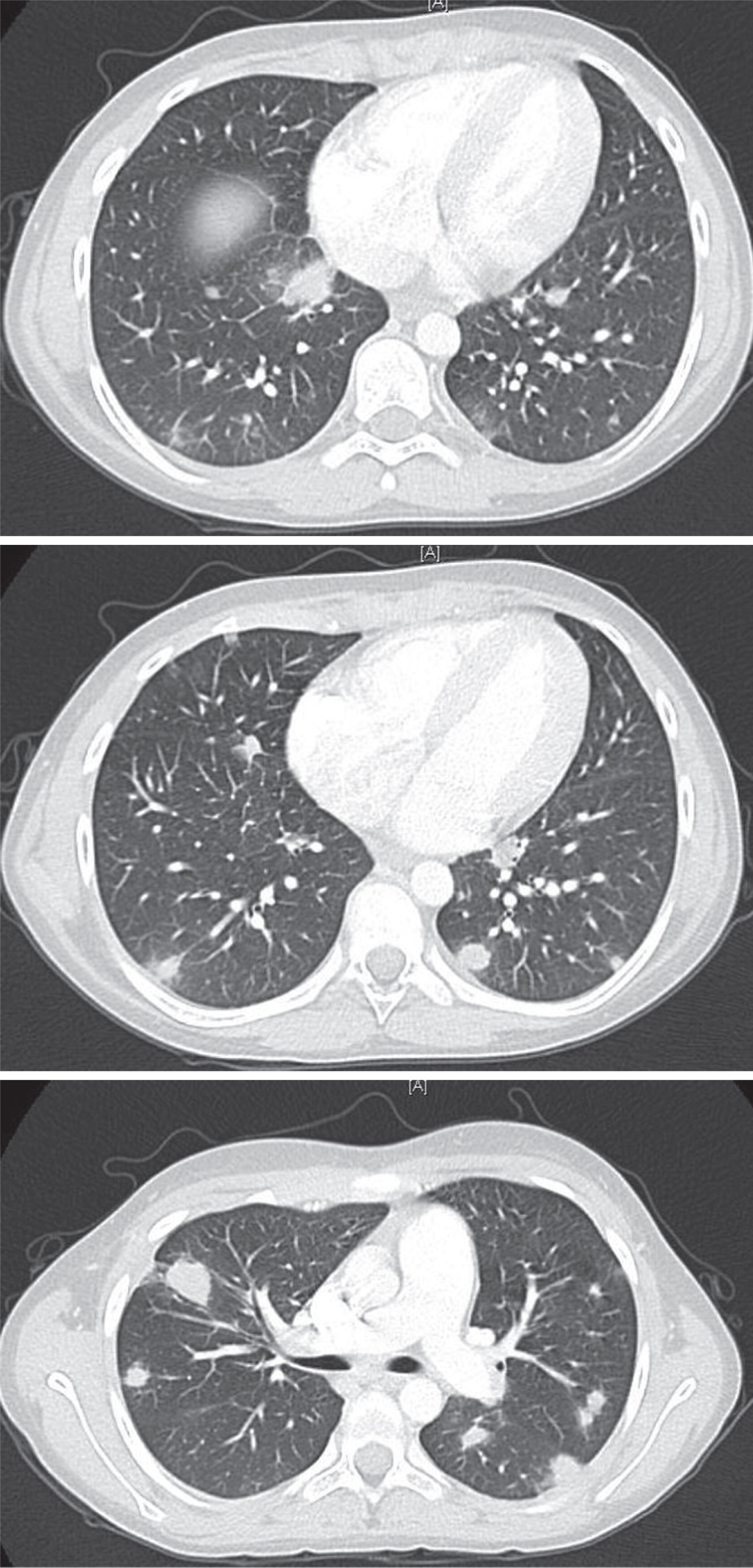
After 1 month, the arthritis and fever relapsed, and the patient developed a new productive cough and pleuritic chest pain. Chest imaging illustrated multiple nodular opacities and enlarged mediastinal lymph nodes (
Figure 1). Induced sputum demonstrated the presence of yeast and pseudohyphae. A follow-up bronchoalveolar lavage confirmed
Aspergillus fumigatus complex. The identification of this unusual infection prompted a request for an Immunology consultation.
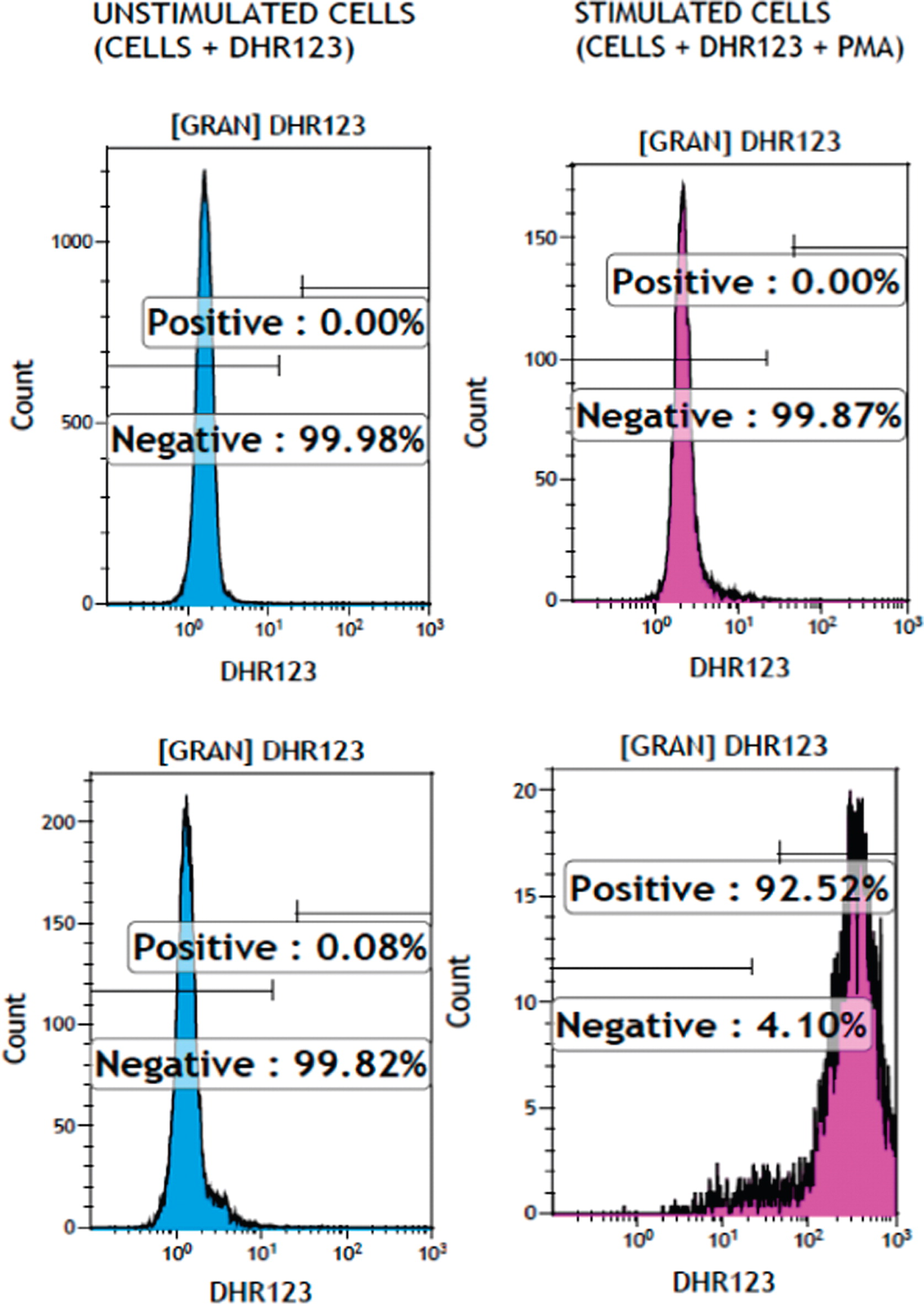
Immunological assessment found that his past medical history was positive for uncomplicated varicella disease at 5 years of age. There was no previous history of pneumonia, lymphadenitis, deep-seated abscess, or osteomyelitis and no prior history of recurrent abdominal pain, vomiting, diarrhea, or blood in the stool. His immunization record was up to date including Bacillus Calmette–Guerin vaccine with no adverse reactions or complications. He had unremarkable lymphocytes subsets (CD3+: 2511 (normal range 800–3500), CD4+: 1250 (normal range 400–2100), CD8+: 1123 (normal range 200–1200), CD19+: 1014 (normal range 200–600), and NK cells: 36 (normal range 70–1200), all in cells/µL), high IgG 19.2 g/L (normal range 6.60–15.30 g/L), high IgE 294 IU/mL (normal range < 200 IU/mL), and normal IgM 1.5 g/L (normal range 0.40–1.5 g/L) and IgA 2.9 g/L (normal range 0.50–2.2 g/L). Specific antibody responses were positive for measles, mumps, rubella and varicella, and protective tetanus titers (0.55 IU/mL). Owing to the finding of
Aspergillus fumigatus, neutrophil oxidative burst function as assessed by dihydrorhodamine flow cytometry based assay (
Figure 2) was abnormally low at 1.26 and 1.48 (normal range 32–300). Genetic testing confirmed a homozygous mutation in the
NCF1 gene (encoding for p47
phox) at c.75_76delGT. The patient received treatment for lung aspergillosis with voriconazole and was placed on cotrimoxazole bacterial prophylaxis. On follow-up, he has continued these treatments and has shown signs of improvement in the size of the lung lesions. He developed intermittent abdominal pain without diarrhea. Enterography MRI and upper and lower endoscopies including biopsies were all normal.