Until 1987, the experience at the Hospital for Sick Children, Toronto, Ontario, with hematopoietic stem cell transplantation (HSCT) using HLA-mismatched donors for the treatment of severe combined immunodeficiency (SCID) was disappointing. Various methods of T-cell depletion of parental HLA-mismatched related donors (MMRD) HSCT had been used with no significant difference in outcomes. The majority of patients who had received HSCT using MMRD-depleted marrow at our institution prior to 1987 died of infections, graft versus host disease (GvHD), or graft loss. Those who survived experienced various degrees of acute and chronic GvHD as well as long-term autoimmune manifestations. Engraftment of lymphocytes was slow and late loss of graft was seen. Large European studies reported approximately 50% survival rate for mismatch donor transplants (
Antoine et al. 2003). However, careful analysis of outcomes according to HLA matching showed a survival rate of 25%–30% for true half-matched donor transplants (
Caillat-Zucman et al. 2004), which are very similar to the results obtained at our institution.
Despite these discouraging results, many centres worldwide adhered to this procedure for 2 more decades. In 1988, with the establishment of the early bone marrow donor registries, Dr. Chaim Roifman pioneered the use of matched unrelated donors (MUD) instead of depleted nonmatched related donors for SCID and other immune disorders. A similar protocol was simultaneously developed in Minnesota by Drs. Alexandra Filipovitch and Ralph Shapiro (
Filipovich et al. 1992). With little historic evidence to support this approach, the protocol was approved at The Hospital for Sick Children and the first patient was enrolled in December 1989. He received his MUD HSCT in April 1990 while admitted to the intensive care unit. He engrafted promptly and was off GvHD prophylaxis and completely immune reconstituted by 8 months of age. The subsequent cases to receive MUD HSCT were a stark contrast to the previous experience with MMRD HSCT as all patients were discharged from the hospital within 3–6 months, engraftment was rapid, and complete and durable and immune reconstitution was maintained for at least more than 2 decades. Complications were limited to GvHD. In the following years, the results remained excellent as initially reported (
Dalal et al. 2000). The key to the durability of this modality of HSCT was the consistency in outcomes obtained in centres around the world (
Grunebaum et al. 2006). This was initially achieved thanks to the work of an exceptionally dedicated group of individuals including Brenda Reid, RN, and clinical and research fellows Drs. David Hummel, Stephen Feanny, Naftaly Meydan, Ilan Dalal, and Shai Cohen.
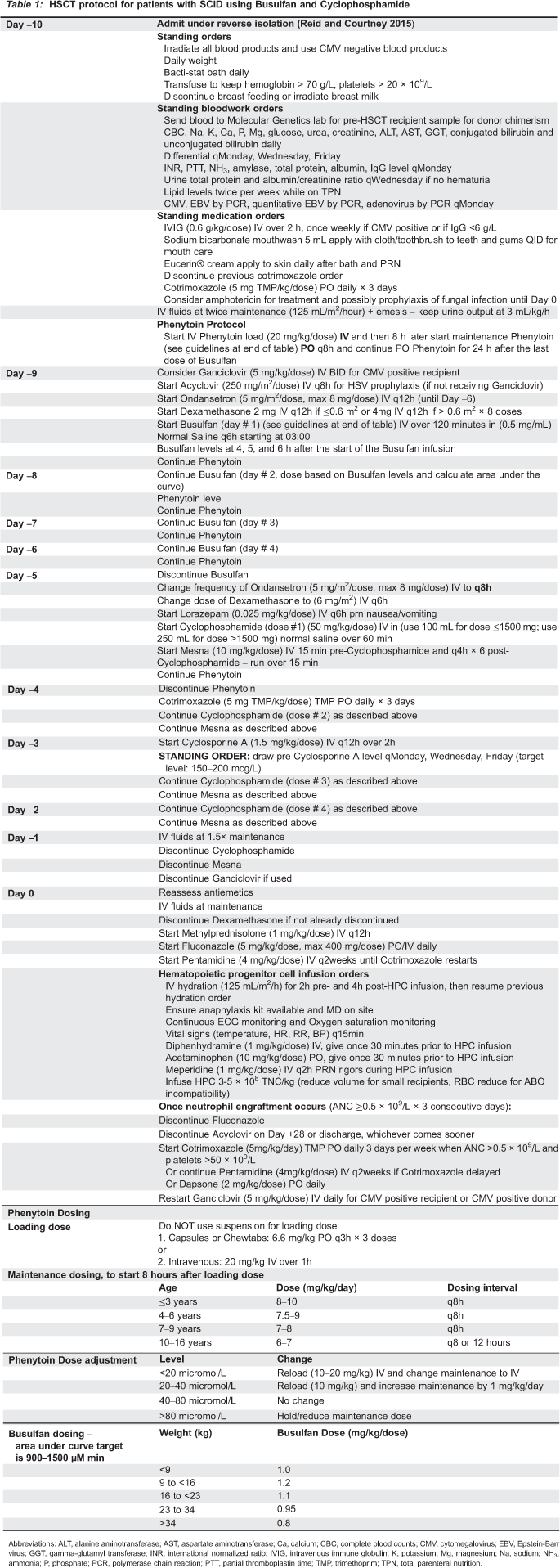
Currently in its third decade, this protocol (see
Table 1) has undergone further improvements and expansions with the help of the highly talented members of the Division of Immunology/Allergy at our institution. Former fellows and current staff, Drs. Adelle Atkinson, Eyal Grunebaum, and Julia Upton, contributed immensely with pre-transplant preparation and counselling with families, establishing an Immunology Transplant Board, and formulating a defined tapering regimen for immunosuppression post-transplant. Many of these activities were supported by an extremely competent group of clinical assistants who have contributed to the care of our patients since 2002 including Drs. Sean Bulley, Karen Mandel, Maria Triassi-Asper, Raz Somech, Amit Nahum, Julia Upton, Fotini Kavadas, and this author.
Although the core of the protocol, such as chemotherapy conditioning and GvHD prophylaxis regimens, remained unchanged, all of the additions and improvements culminated in this solid and balanced treatment guidance for patients who require HSCT and have no HLA-matched related donor. This protocol helped change treatment practice for SCID in many jurisdictions worldwide including recent recommendations by the collaborative group (
European Group for Blood and Marrow Transplantation 2011).
We have not yet reached a full cure for all patients receiving MUD HSCT and there remain challenges to overcome. It is therefore important to view this protocol as a dynamic work in progress with possible modifications according to new evidence.
This protocol is published for the benefit of centres that need guidance in setting up HSCT procedures. Because of a growing number of requests for it, we believe that wide distribution through publication in LymphoSign Journal will meet that need.