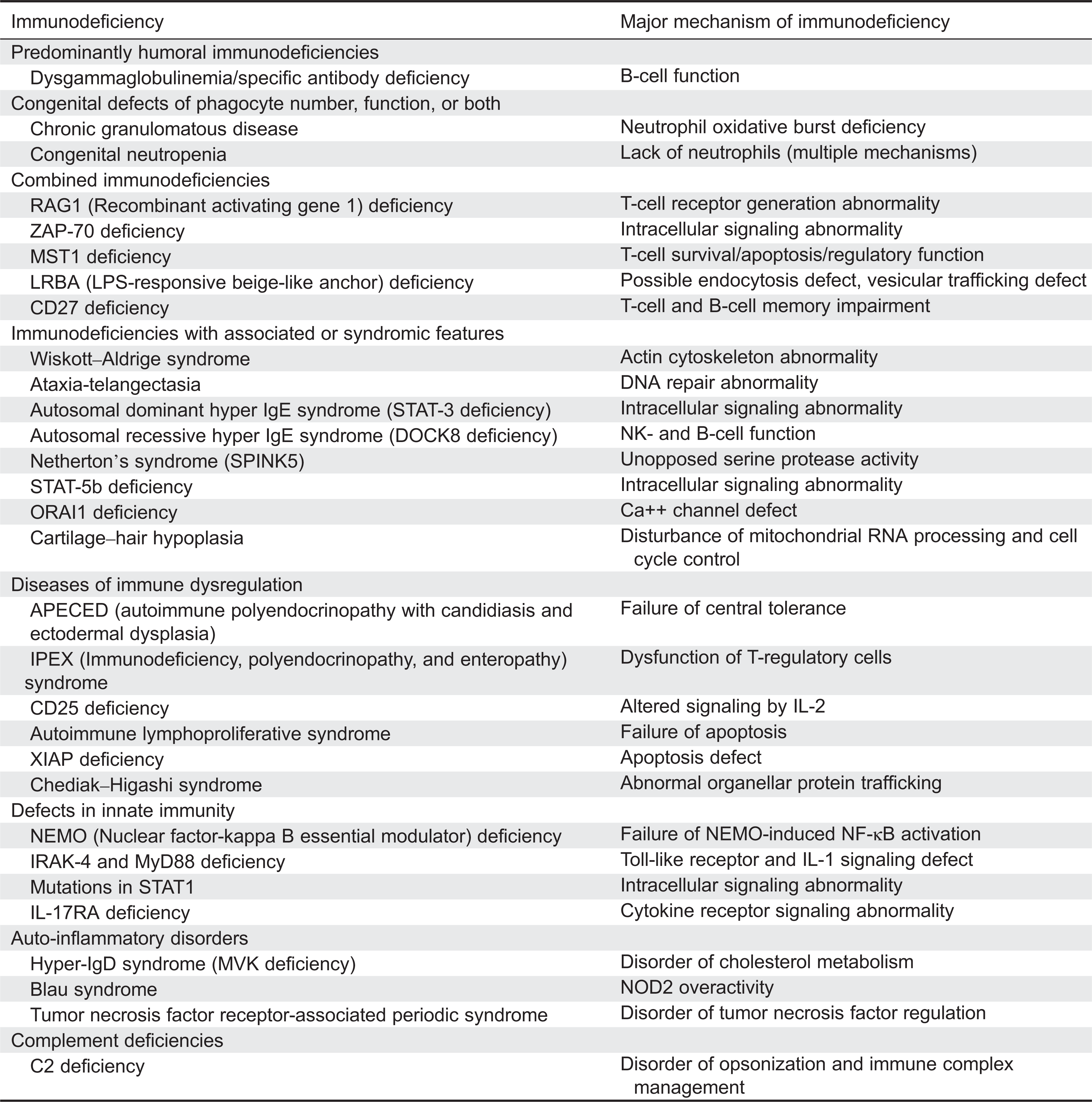
A list of immunodeficiency conditions reported with hypergammaglobulinemia is presented in
Table 1. This table highlights a wide range of immunodeficiencies, including fatal immunodeficiencies, that can have high IgG. They have been mostly organized according to the newest update on the classification of PIDs from the international union of immunological societies expert committee for PID (
Al-Herz et al. 2014) in which the only PIDs specifically listed to be associated with high IgG are mammalian sterile20-like kinase 1 (MST1), also known as serine threonine kinase 4 (STK4) deficiency and autoimmune lymphoproliferative syndrome (ALPS), although many are described as having normal immunoglobulin levels. It is important to also recognize that many more of these PID conditions have been reported with elevated IgG to increase their recognition.
Hypergammaglobulinemia in congenital defects of phagocyte number or function or both
Since the 1950s, there has been recognition that significant immunodeficiencies can occur in the setting of high IgG. Chronic granulomatous disease (CGD) was first described as a disorder in which recurrent infections occur in the setting of hypergammaglobulinemia. This description predated the more advanced testing we have today and was intended to differentiate CGD from Bruton's agammaglobulinemia (
Holland 2013). In CGD there is impairment in NADPH oxidase function. The neutrophils cannot generate superoxide anions and are therefore incapable of creating reactive oxygen intermediates and mounting an appropriate oxidative burst. This impairment leads to a susceptibility to infections with catalase-positive organisms. The NADPH oxidase also contributes to the formation of neutrophil extracellular traps (NETS), extracellular fibres released from the neutrophils that can bind pathogens. These patients can present with recurrent abscesses,
Aspergillus infections, candidal infections, and Crohn's disease-like gastrointestinal disease. CGD can be inherited in an X-linked dominant or an autosomal recessive pattern. X-linked patients have the youngest and most severe presentation and they have mutations in gp91phox, which is specific to the neutrophils. Autosomal dominant patients have mutations in one of the other 4 proteins which compose the NADPH oxidase, p47
phox, p67
phox, p22
phox, or rarely p40
phox. The immune evaluation will typically be normal other than a low neutrophil oxidative burst test, so a high level of suspicion is needed for diagnosis.
Patients with congenital neutropenia have also been found to have high IgG (
Lo et al. 2013). Congenital neutropenia can be caused by multiple different genetic defects including elastase mutations (
Horwitz et al. 2013). These patients have no characteristic clinical features other than neutropenia and infections. Many conditions cause congenital neutropenia (
Boztug and Klein 2013) and those that have been described with hypergammaglobulinemia elsewhere in this review include ALPS, Chediak–Higashi syndrome, and Cartilage–hair hypoplasia. Many causes of congenital neutropenia include specific physical characteristics or associated features that can assist in narrowing down the diagnostic possibilities.
Hypergammaglobulinemia in predominantly antibody immunodeficiencies
Humoral immunodeficiencies are typically associated with hypogammaglobulinemia. However, there are conditions in which the immunoglobulin levels are normal (or high), but they are incapable of mounting a robust specific antibody response against infections. The International PID classification uses the term “Specific antibody deficiency with normal Ig concentrations and normal numbers of B cells” (
Al-Herz et al. 2014) to refer to individuals who have normal immunoglobulins but have recurrent infections and poor responses to vaccines. This terminology does not explicitly describe that some patients with this phenotype can have high, rather than normal, immunoglobulins. Antibody deficiency with normal or elevated immunologlobulins (
Nagumo and Komiyama 2000) recognizes that the immunoglobulins can be high. Some clinical immunologists may also use the term “dysgammaglobulinemia” to refer to these patients. The presence of recurrent infections with high immunoglobulins, but with a poor specific antibody production, is a phenotypic description and represents a compilation of different genetic disorders that will likely be further elucidated in the coming years. Specific antibody deficiency, otherwise known as selective IgG deficiency (
Orange et al. 2012), is another phenotypic condition in which immunoglobulin levels are normal, but there is a poor response to polysaccharide antigens. This description does not currently specify that the IgG can be high. These conditions can exist on their own or as part of a broader phenotype.
Hypergammaglobulinemia in combined T-cell and B-cell immunodeficiencies
A combined immunodeficiency is one in which there is both a T-lymphocyte and B-lymphocyte deficiency.
Many different monogenic combined immunodeficiencies affecting different areas of immune system development have been reported to have elevated IgG. For example, defects in T-cell receptor development, intracellular signaling, regulatory T-cell development, endocytosis, and DNA repair have all been found to cause combined immunodeficiencies as well as high IgG.
T cells recognize antigen through their T-cell receptors. These receptors need to be created from rearranging genomic DNA. Recombinant activating gene (RAG) deficiency leads to an impaired ability to create T-cell receptors. Classically, patients with deficiencies in RAG present with Omenn's syndrome or SCID. However, patients with RAG1 deficiency can have a very variable phenotype. These patients can have prominent autoimmune features and present like patients with granulomatosis with polyangitis (
Avila et al. 2010) or they can have multiple autoimmune cytopenias with IgG above the typical reference range for age (
Henderson et al. 2013;
Chen et al. 2014).
Lymphocytes need to transmit signals from their various cell surface receptors to the nucleus to respond to stimuli. Alterations in lymphocyte signal transduction therefore cause immunodeficiencies, including fatal immunodeficiencies. After the T-cell receptor has bound an antigen, ZAP-70 (Zeta-chain-associated protein kinase 70) is recruited to the CD3 zeta chain and plays a critical role in further downstream signaling. Mutations in ZAP-70 have been described in about 20 families and lead to a severe combined immunodeficiency syndrome characterized by recurrent candidal and viral infections as well as opportunistic infections like
Pneumocystis jiroveci pneumonia (PJP). The immune profile reveals low numbers of CD8 cells, but they can have normal or elevated B cells and IgG with poor specific antibody production (
Fischer et al. 2010). These patients require hematopoetic stem cell transplantation for survival.
A newly described immunodeficiency, MST1 deficiency (also known as STK4 deficiency), is characterized clinically by mild cardiac abnormalities and recurrent bacterial, candidal, and viral infections including Epstein–Barr virus (EBV) and human papillomavirus. They also have a predisposition to lymphoma and autoimmunity. Despite their hypergammaglobulinemia, they have poor B- and T-cell responses (
Abdollahpour et al. 2012). MST1 appears to have a role in regulating regulatory T cells through expression of FOXP3 (
Du et al. 2014) as well as regulating T-cell apoptosis and survival (
Nehme et al. 2012).
Recently, mutations in LPS-responsive beige-like anchor (LRBA) have been described in patients with a variable phenotype of immune dysregulation including inflammatory bowel disease and cytopenias, immunodeficiency, or a combination of all of these features. They have been reported with low, normal, and high IgG, even with the same familial mutation (
Alangari et al. 2012). The exact role of LRBA in the immune system remains to be elucidated but is thought to be involved in endocytosis.
CD27 is a cell marker for memory lymphocytes and is important for NK-cell and T-cell cytotoxicity. CD27 deficiency patients can have a widely variable phenotype within the same family. The patient in the index case initially had elevated immunoglobulins with EBV infection, he then became hypogammaglobulinemic over time and had a good result with IVIG replacement (
van Montfrans et al. 2012). However, his brother died from EBV-related aplastic anemia. Overall, 10 patients have been reported and their clinical phenotype has varied from B memory cell impairment to severe problems such as chronic EBV infection or malignancy (
Parvaneh et al. 2013).
Hypergammaglobulinemia in well-defined syndromes with immunodeficiency
Some immunodeficiencies have strongly associated phenotypic features such as associated neurological impairments, skeletal abnormalities, or other characteristics that can assist in the diagnosis.
Ataxia–telangestasia is an immunodeficiency due to a defect in DNA repair. These patients develop progressive cerebellar defects and have a marked predisposition to malignancy and autoimmunity. Their immune defect is variable and can include humoral immune defects. A large series of patients was described in
Nowak-Wegrzyn et al. (2004) and 13% of the patients had high IgG.
Wiskott–Aldrich Syndrome (WAS) patients are well described as having elevated immunoglobulins, especially IgA and IgE, but also high IgG (
Massaad et al. 2013). The
WAS gene encodes for the WAS protein that transmits signals from the surface of the cell to the actin cytoskeleton. The absence of WAS protein leads to a severe multisystem disorder that includes thrombocytopenia with small platelets, eczema, recurrent infections, and a marked predisposition to develop autoimmunity and malignancy. Many of these patients require bone-marrow transplantation owing to the severity of this condition.
Patients with hyper-IgE syndrome have been described to have high IgG in addition to the more recognized high IgE. In a series of 60 patients, from France, with signal transducer and activator of transcription 3 (STAT-3) mutations from 47 kindred it was found that 27% had high IgG (
Chandesris et al. 2012). This autosomal dominant form of hyper-IgE syndrome is caused by dominant negative mutations in the intracellular signaling molecule STAT-3 (
Sowerwine et al. 2012). It is characterized by recurrent skin and lung infections, skeletal abnormalities such as a propensity to fractures, a high arched palate, and retained primary teeth. Hyper-IgE syndromes can also present with chronic mucocutaneous candidiasis. They have a syndrome of both inefficient infection clearance and disordered inflammation. Patients with STAT-3 mutations have laboratory findings that include increased IgE and eosinophilia and impaired intracellular signaling to a very wide variety of stimuli including the IL-6 pathway and a deficiency of IL-17A and IL-22 producing T cells as well as a deficiency of memory B cells (
Chandesris et al. 2012). Some of these patients require IVIG replacement for their poor specific antibody production.
The autosomal recessive form of hyper-IgE syndrome is caused by mutations in the dedicator of cytokinesis 8 (DOCK8) (
Engelhardt et al. 2009;
Zhang et al. 2009). In the initial description of this condition, 6 of 11 patients had elevated IgG (
Zhang et al. 2009). These patients do not have the characteristic skeletal abnormalities of autosomal dominant hyper-IgE syndrome. They have a rash similar to atopic dermatitis, atopy, recurrent sinopulmonary infections, fungal infections including chronic mucocutaneous candidiasis (CMCC), and markedly increased viral susceptibility. Severe
Herpes simplex, human papilloma virus infections, and mollescum contagiosum have been described. These patients can develop malignancies related to cutaneous viral infection as well as lymphoid malignancies. Commonly, these patients have lymphopenia and eosinophilia and variable specific antibody production. Patients with DOCK8 deficiency are far more likely than patients with atopic dermatitis to have low CD4+, low CD8+, and low memory CD8+ cells (
Janssen et al. 2014). DOCK8 has multiple functions in the immune system including effects on NK- and B-cell function (
Nishikimi et al. 2013). Recently, an impaired ability to affinity switch immunoglobulin and make long-lived immunoglobulin responses to antigen has been demonstrated in mice with defects in this gene (
Randall et al. 2009). Reduced TH-17 cells have also been described. The recognition of DOCK8 deficiency is essential because this condition can be cured with hematopoetic stem cell transplantation (
Su et al. 2011).
Netherton's syndrome occurs owing to mutations in the gene
SPINK5, expressed in epithelial tissue. This mutation alters the LEKT1 protein and allows for unopposed serine protease activity in the skin and results in a condition of severe congenital ichythosis and recurrent infections, especially staphylococcal and viral. As these children grow older they develop atopy. Their immune evaluations typically demonstrate low NK cells, normal or high IgG, and elevated IgE (
Judge et al. 1994).
Children with mutations in
ORAI1 (
Feske et al. 2010) have a syndrome of hypotonia, ectodermal dysplasia, and an immunodeficiency resulting in recurrent severe infections including to opportunistic infections. Their symptoms arise owing to a defect in specialized Ca
++ channel signaling, which has profound effects in the development of multiple systems. The children have been described to have normal lymphocyte numbers but poor activation and normal or elevated IgG.
Another monoallelic immunodeficiency with a recognizable phenotype has been described in patients with STAT-5b mutations. These patients have dysmorphisms, eczema, diarrhea, pneumonias, and severe herpes virus infections. Their failure to thrive is likely mutifactorial, but growth hormone signaling is significantly affected in this condition; therefore, these patients can be identified by low IGF-1, low IGFBP-3, and high prolactin. Their immune evaluation finds normal T-, B-, and NK-cell counts, and they usually have hypergammaglobulinemia and elevated CD45RO counts (
Verbsky and Chatila 2013).
Mutations in the ribonuclease mitochondrial RNA processing endoribonuclease gene result in a disturbance of mitochondrial RNA processing and cell cycle control and cause a highly variable phenotype that can include a combined immunodeficiency as well as short-limbed dwarfism called Cartilage–hair hypoplasia. In a series of 12 patients, the clinical heterogeneity, even within kindred, was stressed and 1 patient was described to have IgG (18.2 g/L) at the age of 2.2 years (
Kavadas et al. 2008)
Hypergammaglobulinemia in diseases of immune dysregulation
Autoimmune cytopenias are a commonly presenting sign of humoral immunodeficiency. As an example, common variable immunodeficiency (CVID) is well described as presenting with immune-mediated thrombocytopenia (
Michel et al. 2004). Persistent or difficult to control autoimmune cytopenias should prompt consideration for PID. By definition, CVID does not have hypergammaglobulinemia, but other immunodeficiencies that have been described with elevated immunoglobulins can have autoimmune cytopenias. One example is DOCK8 deficiency (
Zhang et al. 2009).
As a cause of autoimmune cytopenias, ALPS deserves special mention. Some patients with an ALPS-like phenotype of lymphoproliferation and hypergammaglobulinemia can have B-cell subsets similar to those in (CVID) (
Rensing-Ehl et al. 2010). There is increasing recognition of an overlap of these 2 clinical presentations. Patients with ALPS typically have high IgG, and in fact it is 1 component of the revised criteria (
Oliveira et al. 2010).
A mutation in the autoimmune regulatory element (AIRE) causes derangements in central T-cell tolerance (
Gallo et al. 2013). When T cells are maturing, they need to learn self from non-self. The
AIRE gene regulates the expression of self-antigens in the thymus so that developing T cells have exposure to self-antigens and autoreactive T cells can be deleted. Without this gene, multiple autoimmunity conditions arise. The development of autoantibodies interfering with TH-17 signaling is thought to underlie the susceptibility to candida in this condition (
Puel et al. 2010a). These patients characteristically develop autoimmune polyendocrinopathy with candidiasis and ectodermal dysplasia (APECED). Patients with this condition have been reported to have elevated IgG. A large study of Finnish patients described a subset of 13 patients with skin rashes and of those 5 patients had hypergammaglobulinemia ranging from 24–51 g/L (
Perheentupa 2006).
Mutations in genes essential for regulatory T-cell function can cause combined immunodeficiencies with a predisposition to marked autoimmunity. The
CD25 and
FOXP3 genes both encode for key proteins involved in T-regulatory cell function. Autosomal recessive CD25 deficiency was reported in humans in 1997 as a syndrome in which there is marked infiltration of T cells in the lung, liver, gut, soft tissue, and bone as well as viral, fungal, and bacterial infections. T-cell proliferation in vitro was abnormal but IgG levels were slightly increased (
Sharfe et al. 1997). Mutations in
FOXP3 on the X-chromosome result in a severe disruption of regulatory T cells and result in a syndrome of immunodysregulation, polyendocrinopathy, and enteropathy (IPEX). These patients often have hypergammaglobulinemia (
Verbsky and Chatila 2013). Both of these conditions can be treated with hematopoietic stem cell transplantation.
The X-linked immunodeficiency XIAP deficiency causes a combined immunodeficiency in which the hallmark is susceptibility to the development of hemophaocytic lymphohistiocytosis upon exposure to EBV. In a recent report of a series of 30 patients, 1 patient was described with elevated IgG (
Pachlopnik Schmid et al. 2011). The molecular defect in XIAP confers an increased susceptibility to apoptosis and it interferes with many immunological signaling pathways. Patients with XIAP deficiency are at risk of early death, so this is another very significant immunodeficiency in which the laboratory investigations can show elevated IgG.
Another immunodeficiency that has been described with hypergammaglobulinemia is Chediak--Higashi Syndrome (CHS). In a Venezuelan series, 3 out of 4 of the patients had elevated IgG (
Merino et al. 1983). CHS is caused by mutations in LYST and results in abnormal organelle trafficking. These patients have a recognizable syndrome of disordered immunity in which they typically have partial occulocutaneous albinism and recurrent infections especially to
Staphylococcus aureus,
Streptococcus pyogenes, and
Pneumococcus species. If they survive childhood they can develop significant neurological defects such as peripheral neuropathy. Many patients go on to an accelerated phase in which they develop hemophaocytic lymphohistiocytosis (
Kaplan et al. 2008). Bone-marrow transplantation can cure the hematological and immunological aspects of this condition but not the neurological manifestations (
Tardieu et al. 2005).
Hypergammaglobulinemia in innate immune defects
The innate immune system is so described because it consists of the first line of defense against infections without requiring prior exposure. The innate immune system recognizes preserved antigens in bacteria and viruses. Mutations in IRAK-4 affect signaling through multiple Toll-like receptors and interleukin 1 receptors (
von Bernuth et al. 2012). Recently, mutations in IRAK-4 have also been demonstrated to impair T-cell activation, so there may be a larger role for IRAK-4 in adaptive immunity than currently recognized (
McDonald et al. 2010). A mutation in IRAK-4 was reported to cause hypergammaglobulinemia (
Ku et al. 2007) in a patient with recurrent invasive pneumococcal disease including an episode of meningitis. This patient had good vaccine responses to protein antigens but impaired responses to pneumococcal antigens and low titres to isohemagluttinins. In addition to an infectious history of severe noninvasive and invasive infections from
Streptococcal pneumonia,
Pseudomonas aeriginosa, and
Staph aureus starting at a young age, patients with IRAK-4 deficiency tend to have a blunted inflammatory response to infections, such as the lack of a high fever in the setting of meningitis. In a large series of 48 patients with IRAK-4 deficiency, 12 patients were found to have high IgG (
Picard et al. 2010).
The clinical phenotype of IRAK-4 deficiency is indistinguishable from the phenotype of another innate immune defect, MyD88 deficiency. MyD88 is upstream of IRAK-4 in the Toll-like receptor signalling pathway. Indeed, these patients can also have hypergammaglobulinemia: 4 of 12 patients with MyD88 deficiency had high IgG levels (
Picard et al. 2010). This combined series of IRAK-4 and MyD88 deficient patients demonstrated the severity of these conditions with a mortality of 38%.
Downstream of MyD88 and IRAK-4 is the nuclear factor-kappa B essential modulator (NEMO). Hypomorphic mutations in
NEMO can cause high IgG. In a detailed report of 72 patients (
Hanson et al. 2008), 9 patients were known to have high IgG and another was listed as having high IgG1 and IgG4, so he too likely had total elevated IgG. This series reported that 2 of 10 patients died in childhood. Children with NEMO mutations have a complex phenotype. Some, but not all children, have ectodermal dysplasia and they have variable immune defects. Additionally some children have a susceptibility to mycobacteria and they may have antibody deficiency or a hyper-IgM syndrome.
Some mutations in the transcription factor, STAT-1, alter signaling of cytokines including that of IFN-gamma, IL-6, and IL-21 reduce IL-17 producing T cells and can cause CMCC, recurrent viral infections, enteropathy, mycobacterial infections, and multiple endocrine autoimmunity (
Uzel et al. 2013). These patients can have elevated immunoglobulins with poor antibody production among other immune abnormalities such as lymphopenia. Importantly, high IgG is not reassuring at all in STAT1 mutations. A child with a heterozygous mutation in the DNA binding domain of STAT1 was reported to have high IgG, CMCC, and a combined, progressive immunodeficiency so profound that death occurred in childhood (
Sharfe et al. 2014).
Another cause of CMCC causing hypergammaglobulinemia has been described. A mutation in IL-17RA was identified in a boy with
C. albicans and
S. aureus dermatitis born to consanguineous parents. This mutation caused deficiency of IL-17RA and completely abolished responses to IL-17A and IL-17F (
Puel et al. 2011).
Hypergammaglobulinemia in auto-inflammatory disorders
Increasing understanding of the inflammasome and defects in its regulation has shed light on the autoinflammatory conditions. Autoinflammatory conditions include the classic periodic fever syndromes, as well as some newer conditions described such as DIRA (deficiency of the IL-1 receptor antagonist). Many autoinflammatory conditions have been reported to have hypergammaglobulinemia (
Kastner et al. 2010). These conditions are well known to have high IgG and not classic primary immunodeficiencies, so they will not be discussed in detail. Tumor necrosis factor receptor-associated periodic syndrome (
Cantarini et al. 2012), mutations in mevalonate kinase (
Bader-Meunier et al. 2011), proteasome associated disorders (
Goldbach-Mansky 2012), and Blau syndrome (
Sfriso et al. 2012) have all been described with hypergammaglobulinemia.
Schnitzler syndrome is a considered an autoinflammatory syndrome with a constellation of recurrent fevers, urticarial-like rash, joint inflammation, hepatosplenomegaly, and lymphadenopathy. Although these patients typically have an elevated monoclonal IgM, there are IgG variants (
Schuster et al. 2009).