The male patient was born at term to nonconsanguineous parents of English descent after a normal pregnancy and delivery. From 2 months of age, the patient suffered intractable profuse watery and bloody diarrhea with up to 30 bowel movements a day. There was no improvement in stooling despite changes in formula feeds, performed because of presumed food allergy. At 2 months of age the patient experienced respiratory syncytial virus bronchiolitis that resolved. At 8 months of age the patient experienced rotavirus enteritis. The patient was admitted to hospital at 9 months of age for protracted diarrhea. He was found to suffer from failure to thrive, hypertension, extensive eczema, lymphadenopathy, ascites, hypotonia, and motor developmental delay. There was no history of immunodeficiency, autoimmunity, or bowel disease in the parents or their extended families, apart from a maternal second cousin with type 1 diabetes mellitus.
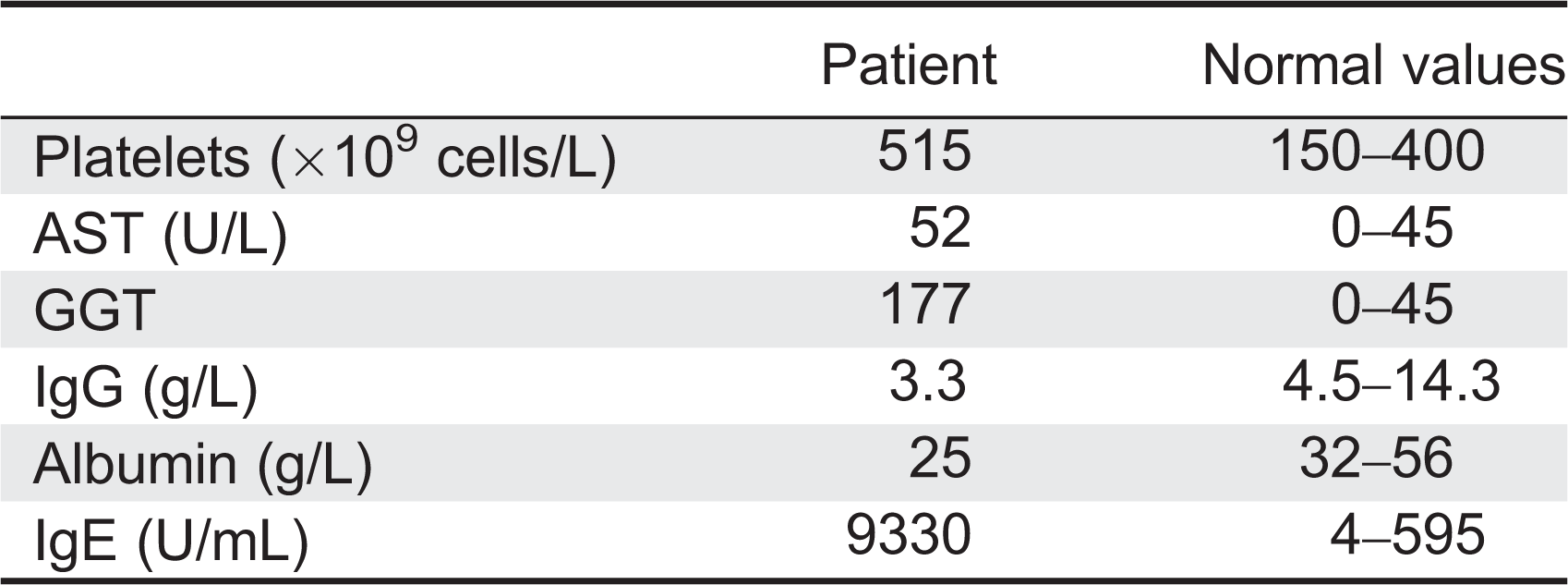
Laboratory investigations at admission revealed hypoalbuminemia, massive proteinuria, and microscopic hematuria. Complete blood count was normal apart from elevated platelet count (
Table 1). Liver enzymes were mildly deranged. Flow cytometry analysis showed normal numbers of CD20+, CD3+, CD4+, CD8+, and NK cells. T-cell receptor excision circles, indicative of new thymus emigrants, were within normal range for the age. In-vitro responses of lymphocytes to stimulations with mitogens and antigens, serum IgA and IgM levels, as well as specific antibody titers were within normal ranges. Serum IgG was slightly reduced, possibly reflecting the protein loss, whereas IgE concentration was markedly elevated (
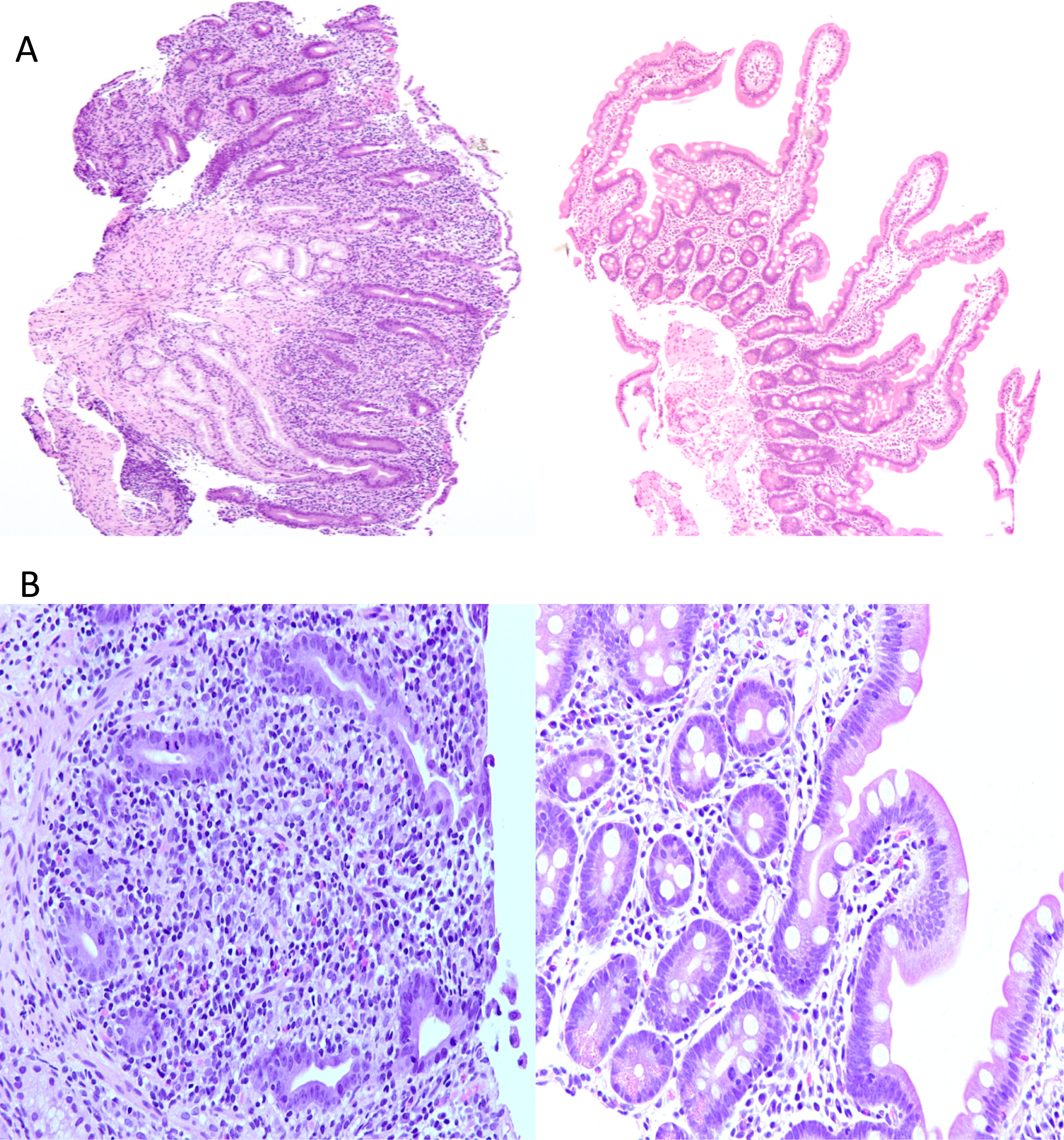
Table 1). Neutrophil oxidative burst index and complement levels were also normal. Auto-antibodies against islet cells, glutamic acid decarboxylase (GAD), insulin, thyroid peroxidase (TPO), thyroid receptor, adrenal, nuclear, smooth muscle, mitocondrial, and liver–kidney microsome (LKM) were all negative. Duodenal biopsies showed complete villous atrophy, crypt hyperplasia, and an inflammatory infiltrate in the lamina propria (
Figure 1A) as well as loss of the normal villus brush border and absence of goblet cells (
Figure 1B). Crypt abscesses with apoptotic epithelial cells were evident (
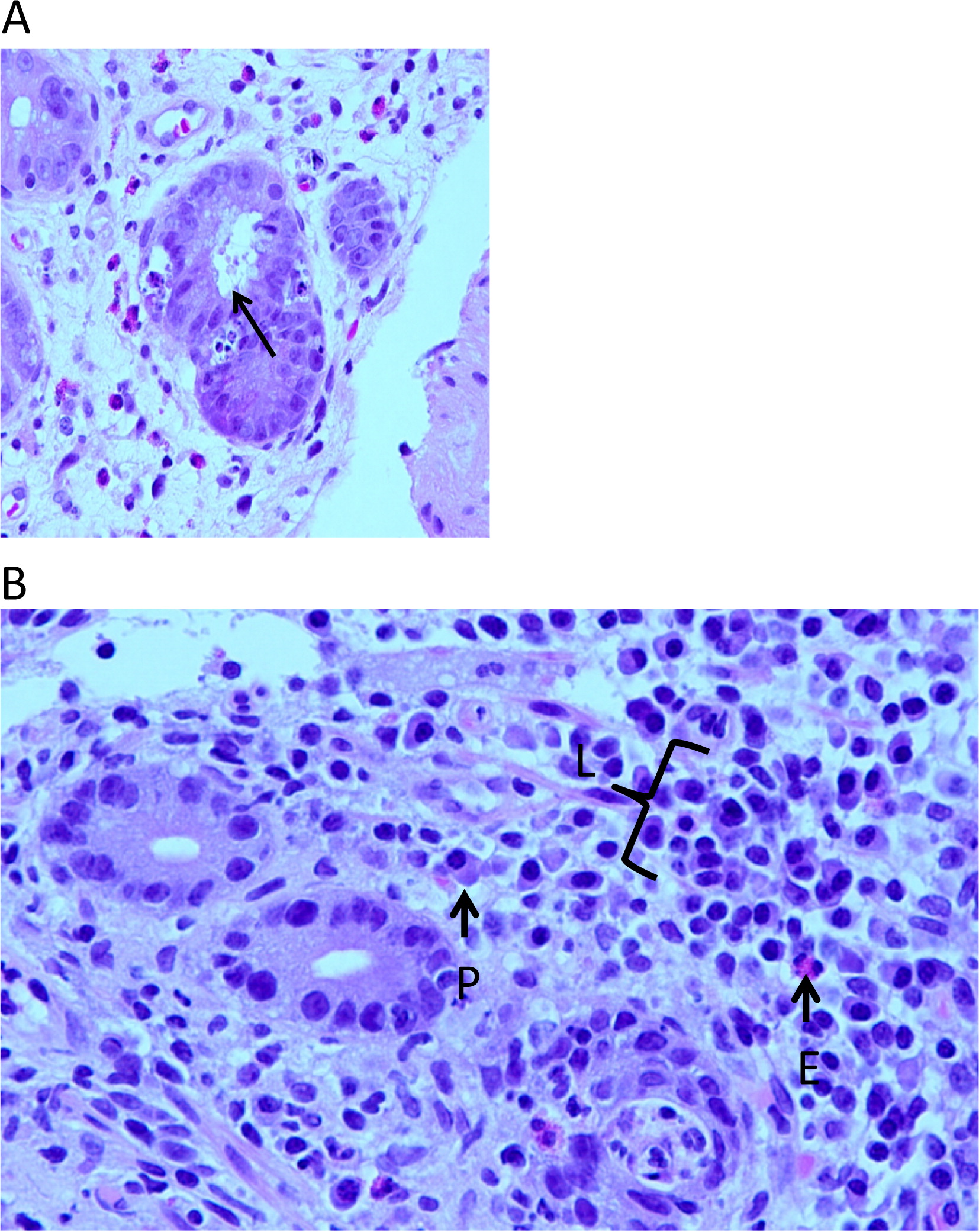
Figure 2A), whereas the lamina propria infiltrate consisted predominantly of lymphocytes with occasional plasma cells and eosinophils (
Figure 2B). Immuno-staining for CD3 demonstrated an extensive T-cell infiltrate (
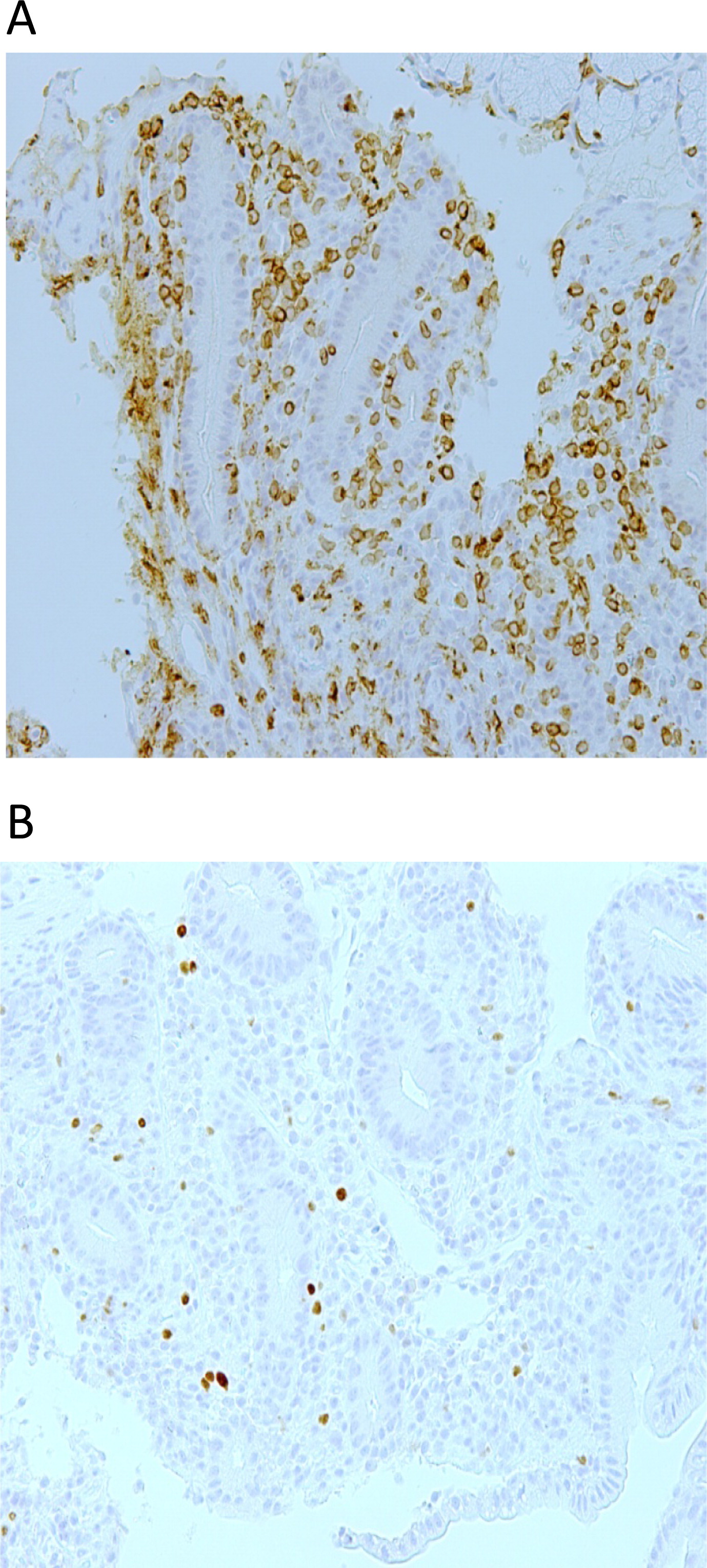
Figure 3A) with the presence of FOXP3+ cells (
Figure 3B). These findings suggested an autoimmune colitis. Biopsies from stomach, colon, sigmoid, and rectum were unremarkable. A renal biopsy showed membranous glomerulonephritis (
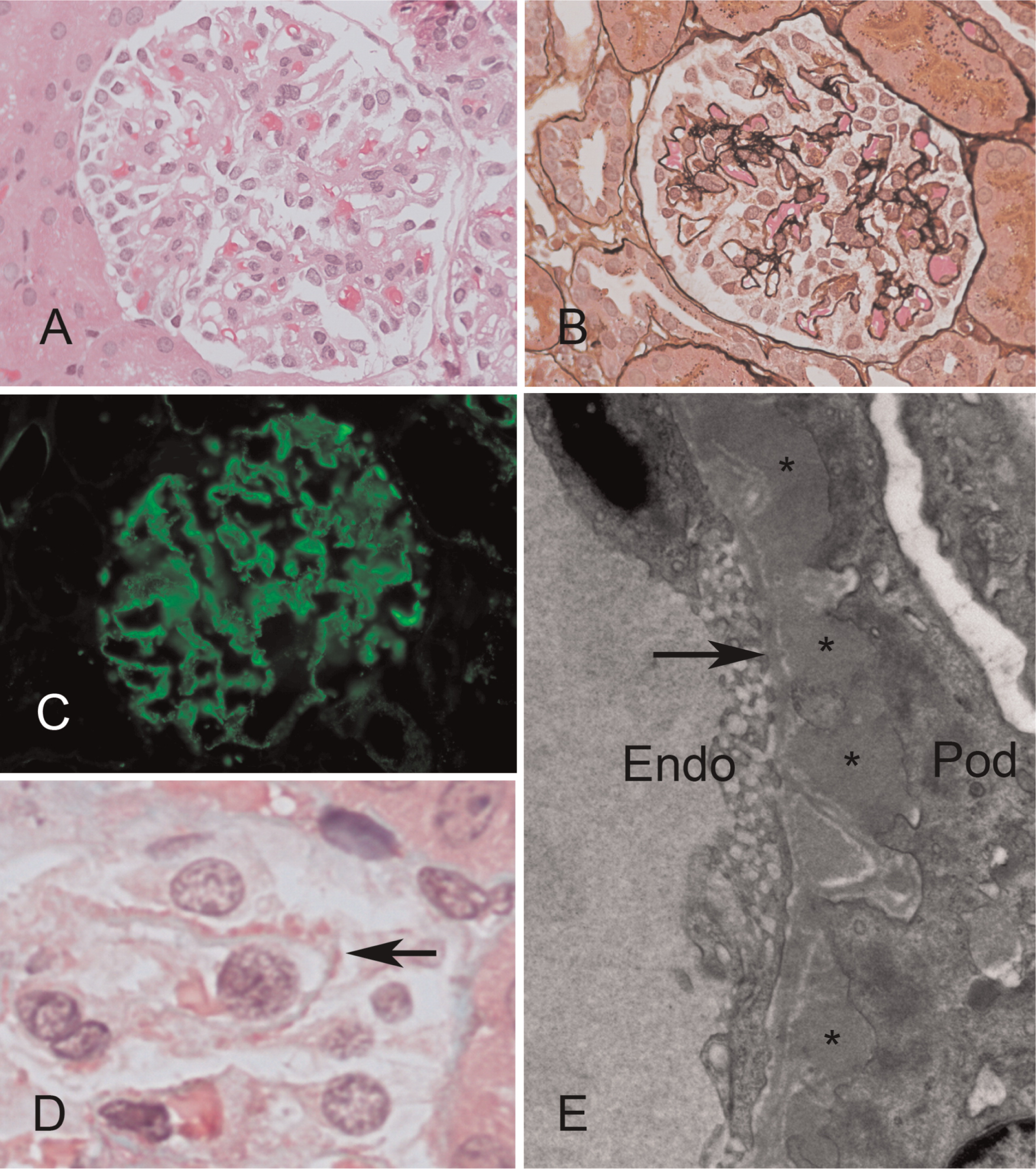
Figure 4). Sanger sequencing of the
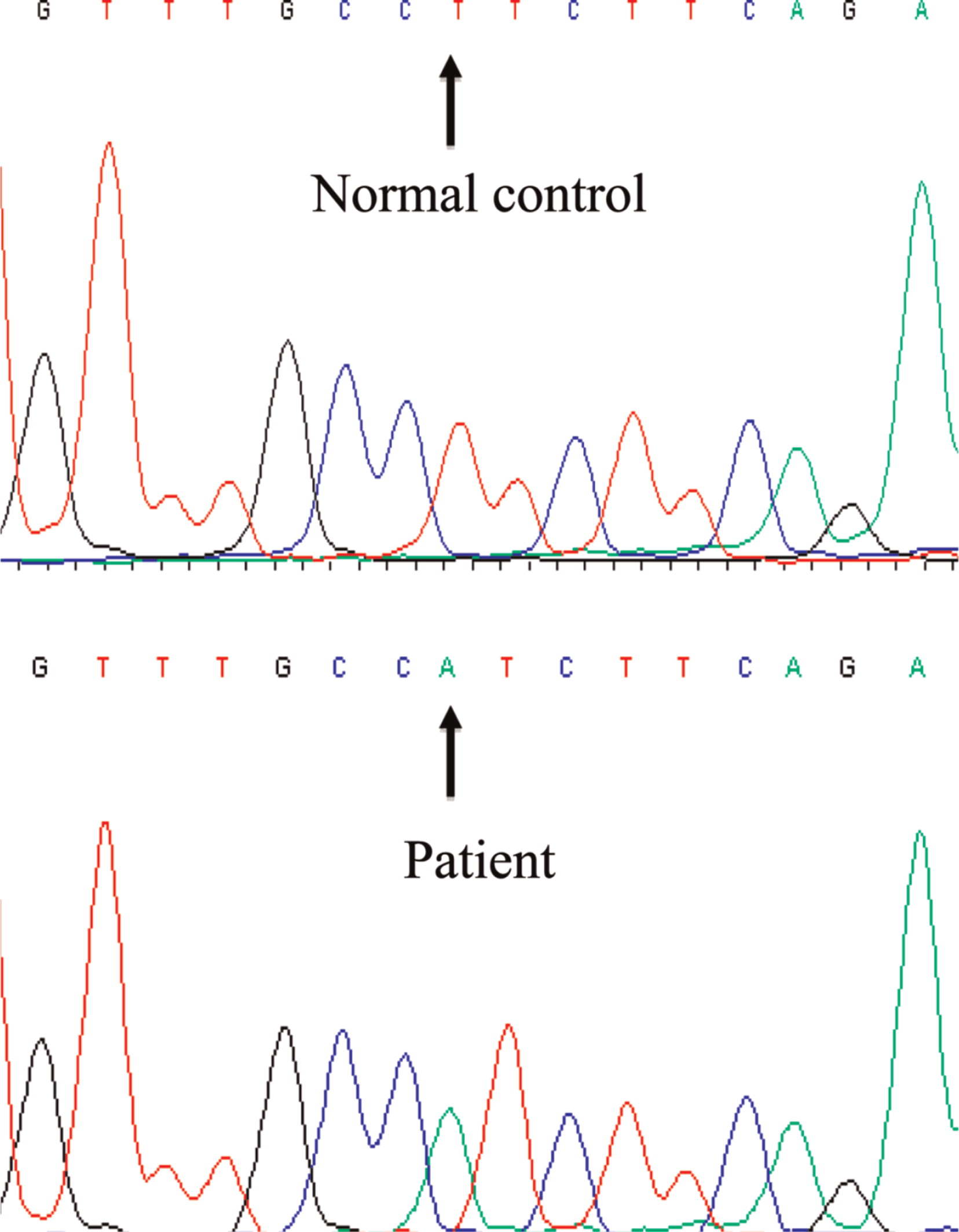
FOXP3 gene revealed a novel hemizygous T1117A substitution (
Figure 5) putatively causing an exchange of isoleucine for phenylalanine at amino acid 373, an area in the FKH domain prone to mutations (
D'Hennezel et al. 2012). The patient's mother was heterozygous for the mutation.
Despite prolonged immune suppression with methylprednisolone and cyclosporine, the diarrhea and proteinuria persisted. At 16 months of age the patient received bone marrow HSCT from a female who was CMV negative and 10 out of 10 HLA MUD. Conditioning consisted of 5 mg/kg busulfan for 4 days followed by 50 mg/kg cyclophosphamide for 4 days, a regimen similar to that used by us for other forms of primary immune deficiencies (
Grunebaum et al. 2006). The patient tolerated the conditioning regimen well except for transient elevation of liver transaminases. The patient developed grade IV skin graft-versus-host disease despite methylprednisolone and cyclosporine prophylaxis that was treated with methylprednisolone pulse followed by mycophenolate mofetil and sirolimus. The diarrhea improved with reduced frequency of stools within 1 month of HSCT and normalization of stool frequency and consistency a month later. The nephropathy improved dramatically; the urinary albumin–creatinine ratio declined from a high of 1139.5 at presentation (normal < 3.5) to 12 within 3 months of HSCT and was normalized by 9 months.
At 3.5 years after HSCT, the patient is eating normal solid foods without any evidence of colitis. There are also no indications of nephritis, endocrinopathy, autoimmunity, neurological abnormalities, or developmental delay. He has not had any significant infections, lymphocyte sub-populations numbers are normal, and FISH analysis with X and Y chromosome probes of the patient's lymphocytes demonstrates complete (100%) donor chimerism.