Cardiovascular abnormalities in primary immunodeficiency diseases
Abstract
Introduction
Predominantly T lineage defects
Omenn syndrome
Calcium channel deficiency
Chronic mucocutaneous candidiasis
STK-4 deficiency
Lymphocyte-specific protein tyrosine kinase deficiency
DNA repair defects
Ataxia–telangiectasia
Nijmegen breakage syndrome
Predominantly antibody deficiencies
Common variable immunodeficiency
TWEAK deficiency
Predominantly defects of neutrophils/macrophages number and function
Severe congenital neutropenia type 4/G6PC3 deficiency
Barth syndrome
Cohen syndrome
Shwachman–Diamond syndrome
WHIM syndrome
MonoMAC syndrome
Chronic granulomatous disease
Well-defined syndromes with immunodeficiency
DiGeorge syndrome
CHARGE syndrome
Wiskott–Aldrich syndrome
Roifman syndrome
Schimke immuno-osseus dysplasia
Hyper-IgE syndromes
Immunodeficiency with centromeric instability and facial anomalies
Hepatic veno-occlusive disease with immunodeficiency
Facial dysmorphism, immunodeficiency, livedo, and short stature (FILS syndrome)
Disease of immune dysregulation
X-linked lymphoproliferative disease
FADD deficiency
Aicardi–Goutières syndrome
Autoinflammatory disorders
HOIL1 deficiency
Conclusion
REFERENCES
Appendix A1: Cardiac and vascular abnormalities associated with primary immunodeficiency disorders.
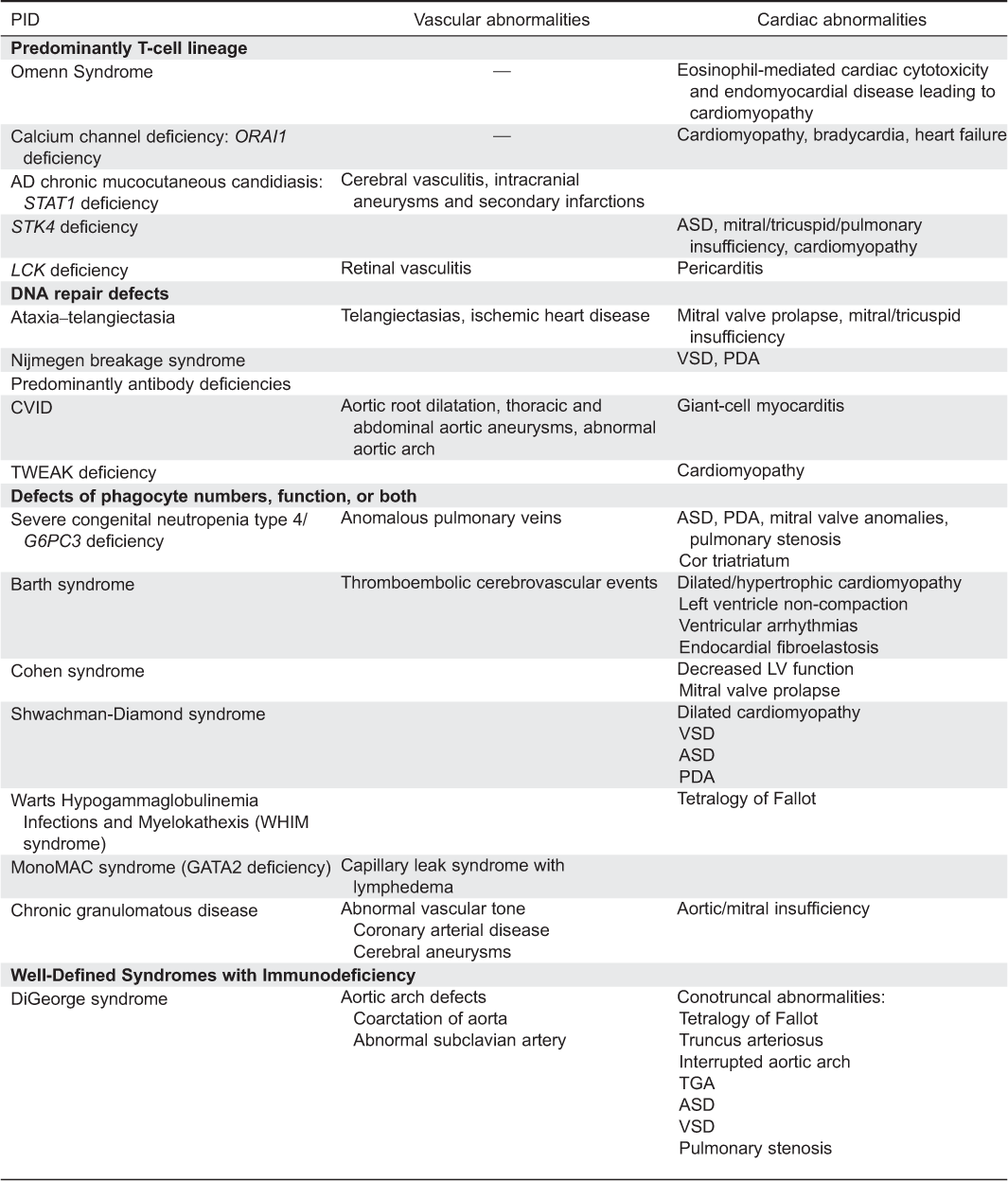
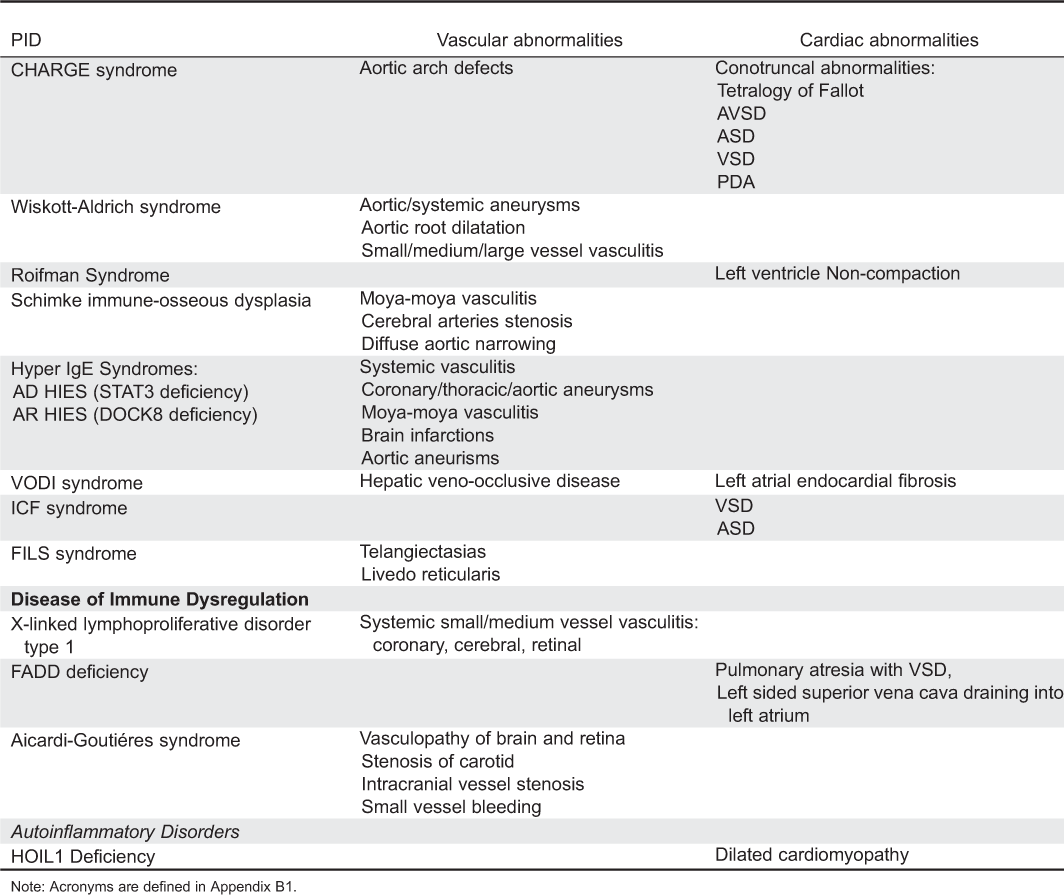
Appendix A2: Primary immunodeficiency disorders associated with cardiac abnormalities.

Appendix A3: Primary immunodeficiency disorders associated with vascular abnormalities.
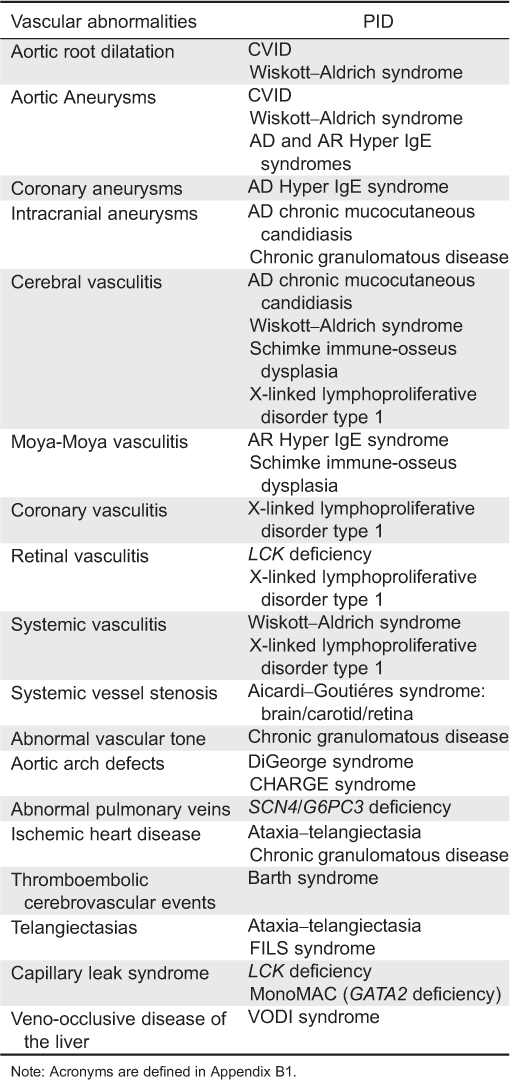
Appendix B1: Acronmyms

Information & Authors
Information
Published In

History
Authors
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
There are no citations for this item
View Options
View options
Login options
Check if you access through your login credentials or your institution to get full access on this article.


