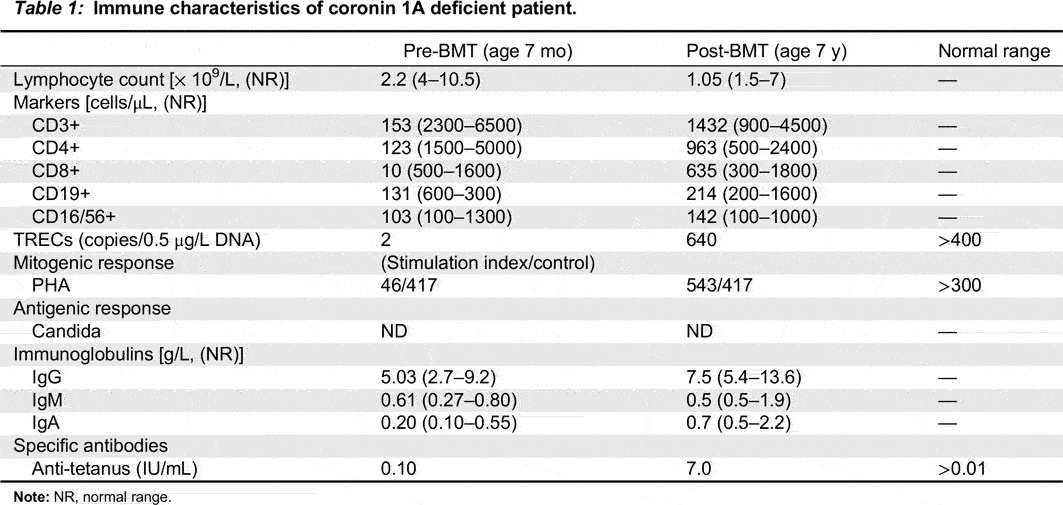
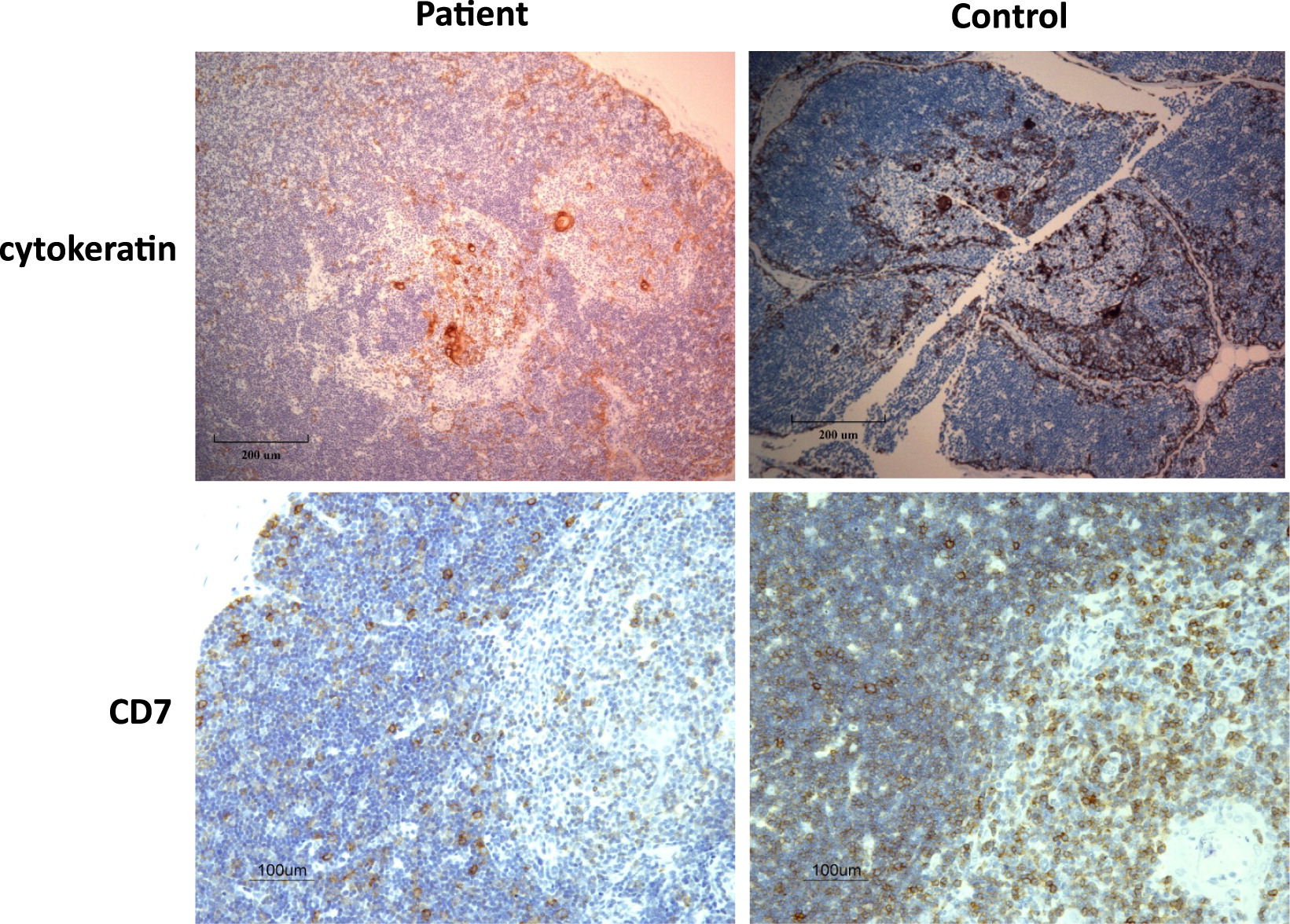
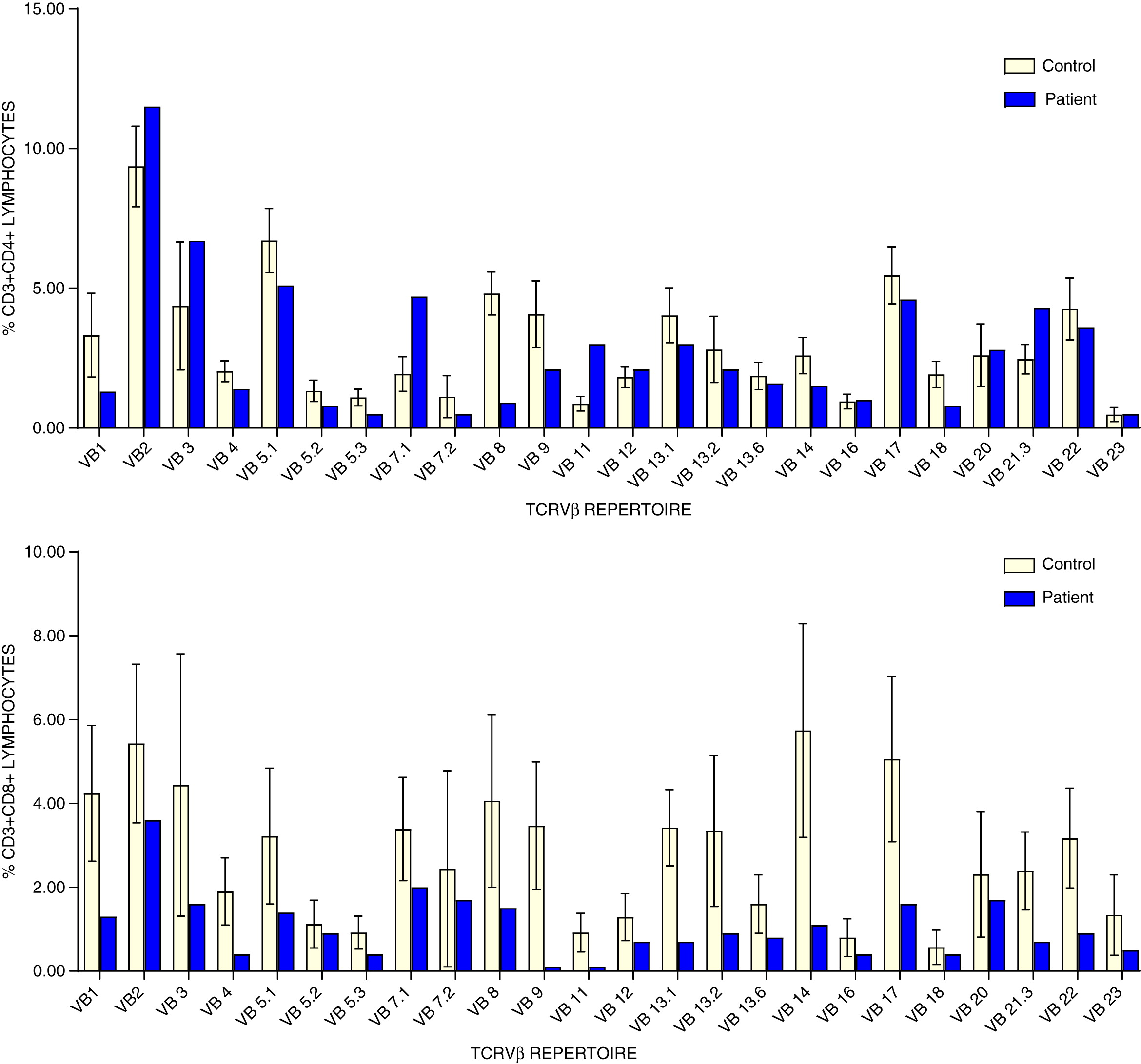
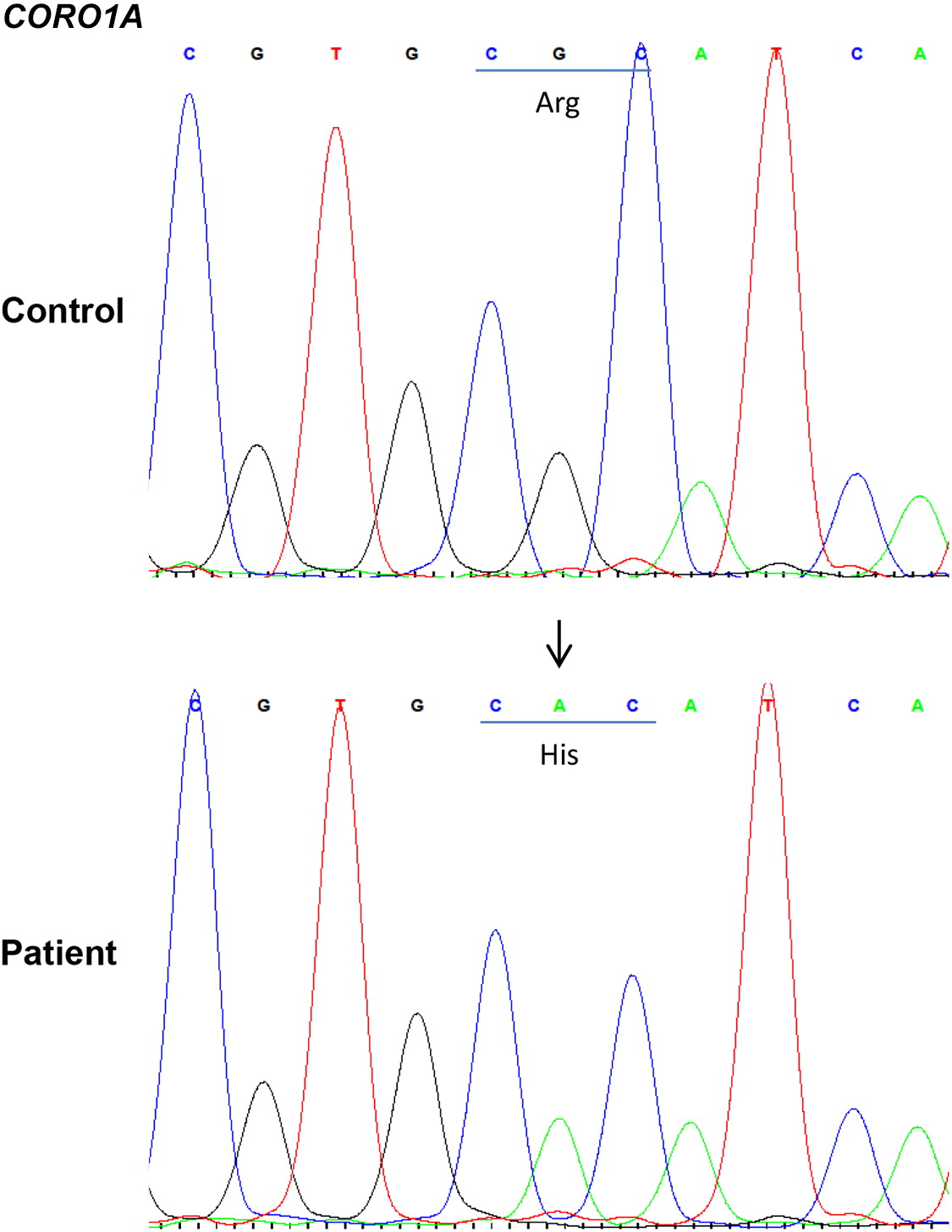
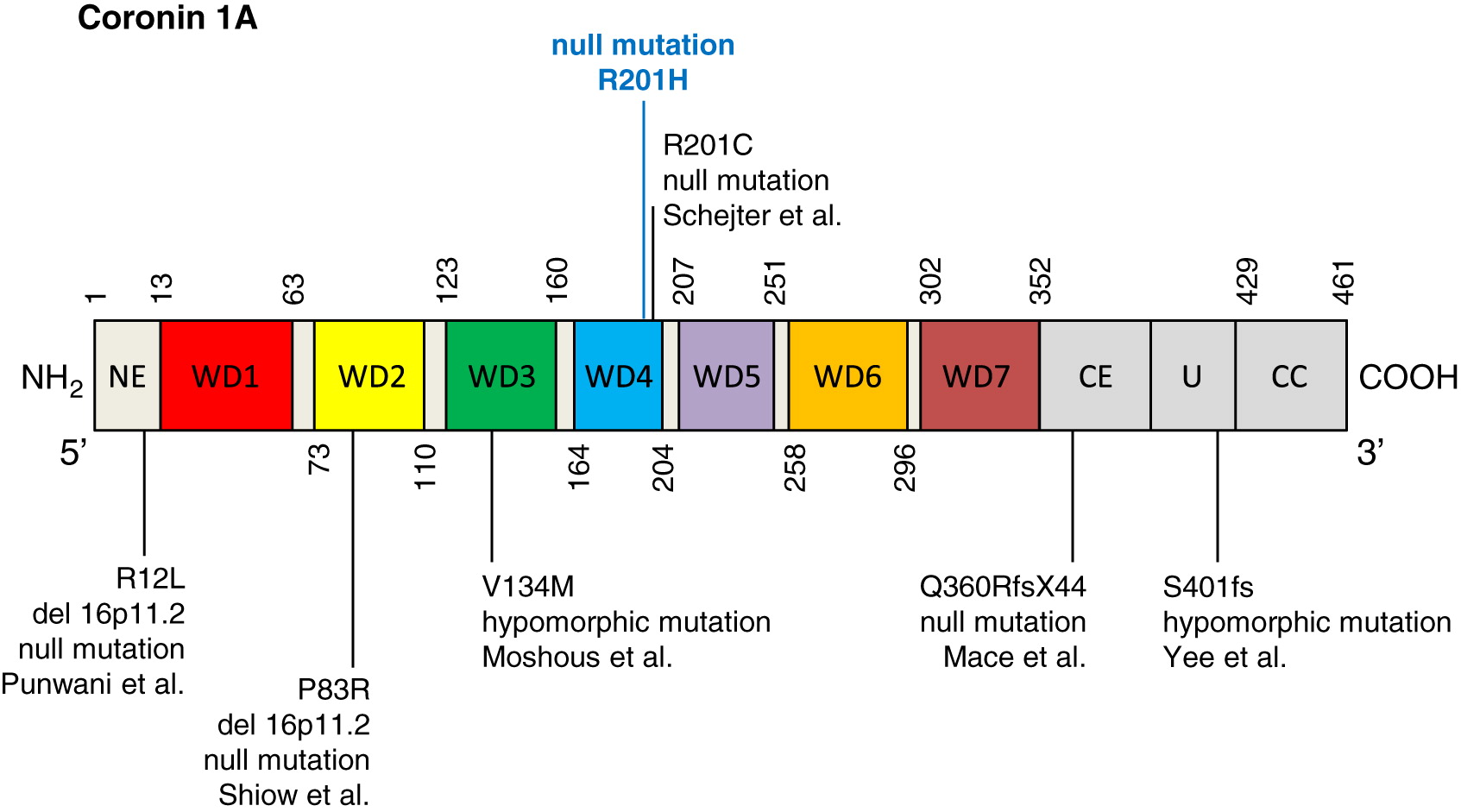
Coronin 1A is a key regulatory protein involved in cytoskeletal remodeling and calcium signaling (
Rybakin and Clemen 2005;
Mueller et al. 2008), and is expressed in a number of hematopoietic cells, including lymphocytes, macrophages, and NK cells (
Oku et al. 2003). Part of a larger family of highly conserved actin regulatory proteins (
Xavier et al. 2008), the structure of coronin 1A comprises: (
i) an amino tryptophan-aspartate (WD) repeat-containing region forming a beta propeller—a motif involved in plasma membrane binding; (
ii) a unique region; and (
iii) a leucine zipper coiled-coil domain that is required for oligomerization and cytoskeletal association (
Gatfield et al. 2005;
Kammerer et al. 2005).
The 46 amino acid, 57 kDa actin binding protein is encoded by the
CORO1A gene, located on chromosome 16p11.2 (
Suzuki et al. 1995). Essential roles for coronin 1A have been reported in cell survival, migration, phagocytosis, vesicular trafficking, and signal transduction (
Rybakin and Clemen 2005;
Foger et al. 2006;
Mueller et al. 2008,
2011). In murine studies, loss of coronin 1A expression is associated with T lymphocytopenia, due in part to aberrant T cell receptor mediated proliferation and failure of T cells to progress through the cell cycle (
Foger et al. 2006;
Haraldsson et al. 2008;
Mueller et al. 2008;
Mugnier et al. 2008;
Shiow et al. 2008). Dysfunctional CD4+ T helper cell and associated IgG responses have also been reported (
Tchang et al. 2013), while B and NK cells remain at normal levels. In humans, null mutations in
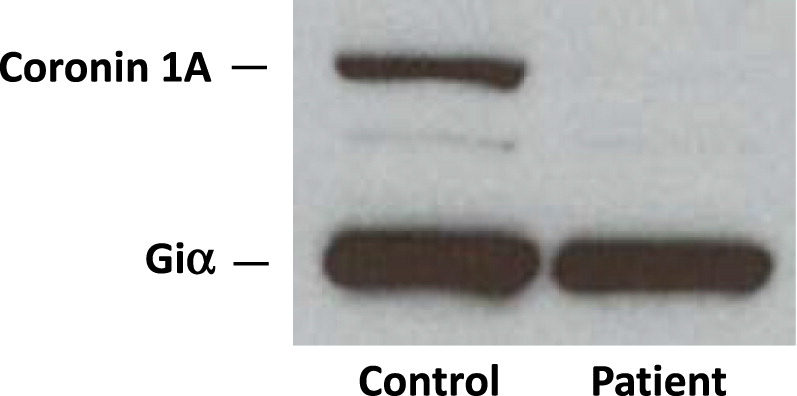
CORO1A resulting in complete absence of coronin 1A expression are associated with a severe combined immunodeficiency phenotype (
Shiow et al. 2008), while hypomorphic mutations lead to somewhat milder immunological manifestations (
Moshous et al. 2013;
Yee et al. 2016). In all cases described so far, patients experienced recurrent infections, had markedly reduced naïve peripheral T cells, and impaired T cell responses to mitogens (
Moshous et al. 2013;
Mace and Orange 2014). All but 3 patients showed failure to control Epstein-Barr virus (EBV) predisposing to B cell lymphoproliferation and lymphoma (
Yee et al. 2016;
Dinur Schejter et al. 2019). Interestingly, the thymus of coronin 1A deficient patients appears normal, indicating that loss of naïve T cells may be due to defects in cell survival. Variability in humoral function, B and NK cell counts, as well as T cell responses to mitogens and antigens have been described among affected individuals.
To date, only 2 patients with coronin 1A deficiency are reported to have received hematopoietic stem cell transplantation (HSCT). The first had a human leukocyte antigen (HLA)-matched unrelated donor transplant at the age of 4 years (
Shiow et al. 2009). Although the procedure was successful, a full account of long term outcome has not been provided. The second patient received 6/6 HLA-matched parental bone marrow, however, died at 4 months post-transplant due to respiratory failure (
Moshous et al. 2013).
Here, we describe a novel mutation in coronin 1A in a patient of Canadian Inuit origin. The patient successfully underwent HSCT using a HLA-matched unrelated donor and achieved long term engraftment and solid immune reconstitution.