Histamine, histamine receptors, and anti-histamines in the context of allergic responses
Abstract
Introduction
Histamine in the context of atopic disease
Receptor structure
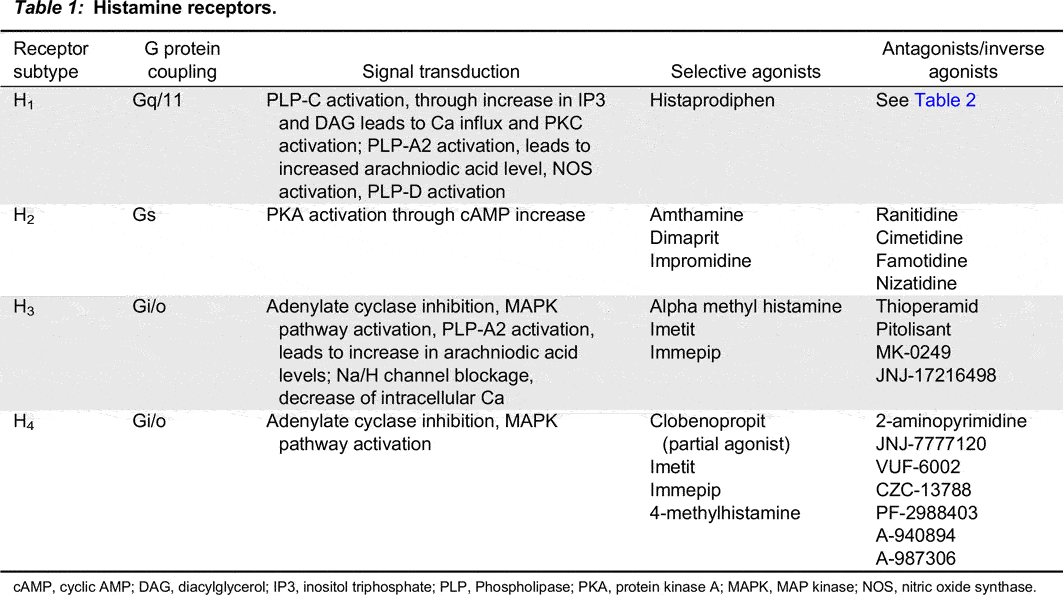
cAMP, cyclic AMP; DAG, diacylglycerol; IP3, inositol triphosphate; PLP, Phospholipase; PKA, protein kinase A; MAPK, MAP kinase; NOS, nitric oxide synthase.
Histamine receptor expression and function
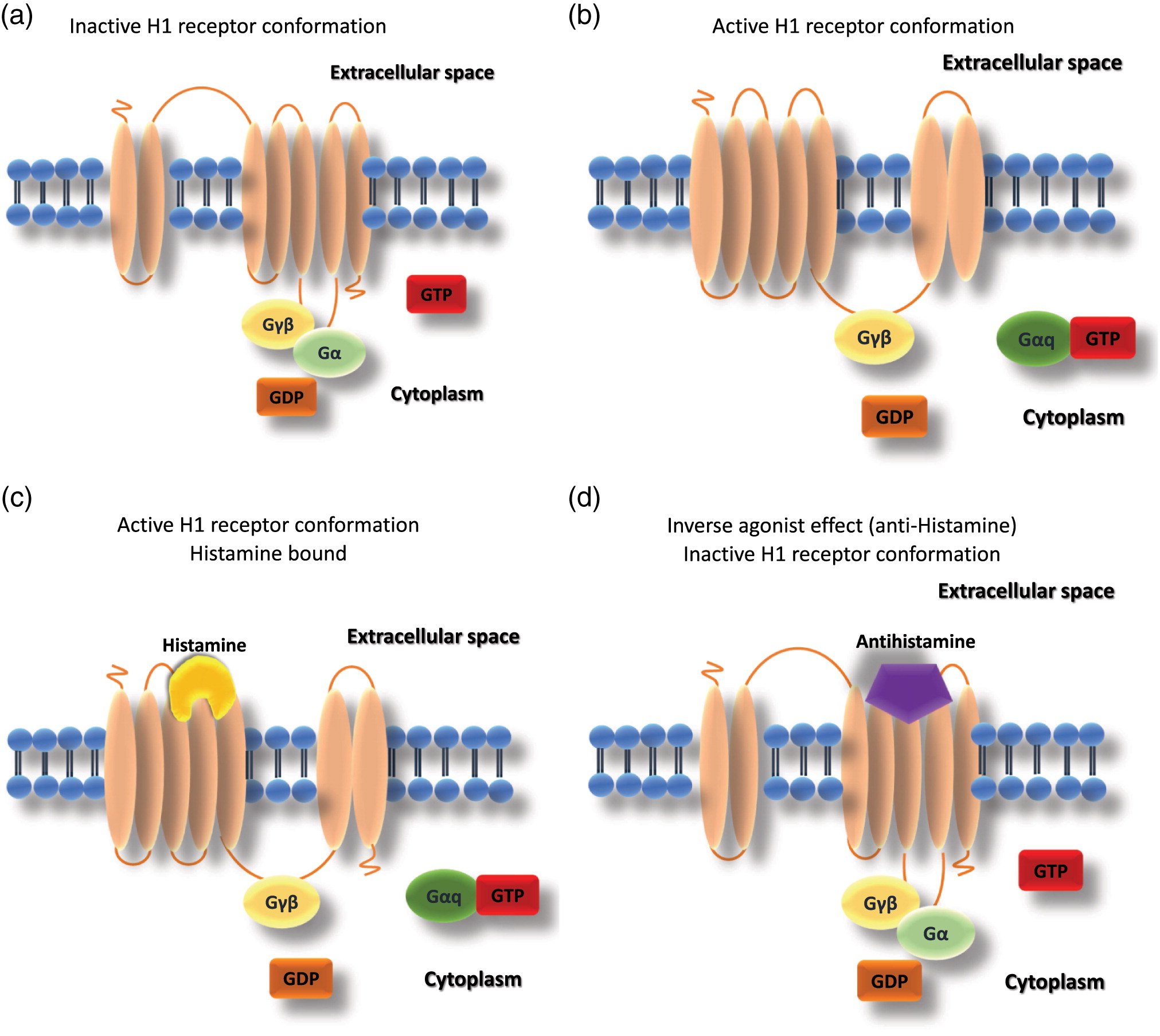
Pharmacological blockade of histamine function via antihistamines
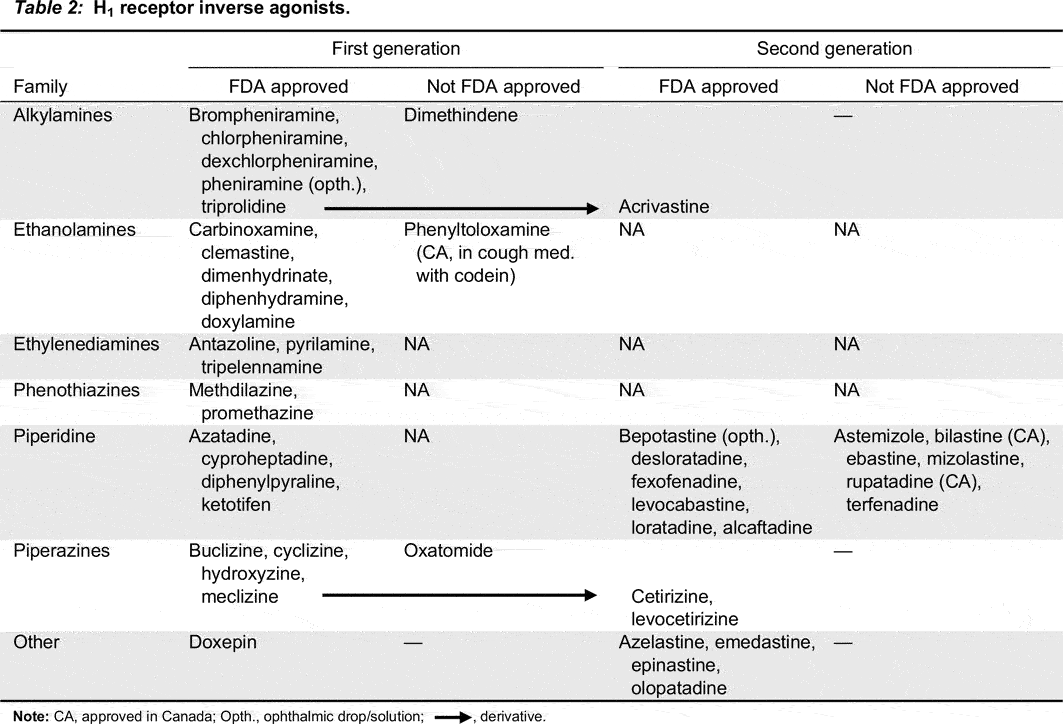
Note: CA, approved in Canada; Opth., ophthalmic drop/solution;, derivative.
Pharmacokinetics and side effects of approved antihistamines
Bioavailability
Selectivity
Antihistamines in atopic disease
Chronic urticaria
Allergic rhinitis
Allergic conjunctivitis
Asthma
Food allergy
Novel applications of antihistamines
Frequently used non-sedating H1 antihistamines
Cetirizine and levocetirizine
Loratadine and desloratadine
Bilastine
Fexofenadine
Discussion
REFERENCES
Information & Authors
Information
Published In

History
Copyright
Authors
Funding Information
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
There are no citations for this item
View Options
View options
Login options
Check if you access through your login credentials or your institution to get full access on this article.


