Introduction
The DNA-binding protein Signal Transducer and Activator of Transcription 1 (STAT1) is a member of a family of transcription factors that plays a major role in different and diverse functions of the cell. Following activation by interferon-gamma or interferon-alpha, STAT1 protein is phosphorylated and forms dimers. It then translocates into the nucleus, binds to DNA, and initiates its effector function (
Darnell et al. 1994;
Stark et al. 1998;
Levy and Darnell 2002). Different genetic mutations in
STAT1 resulting in different clinical phenotypes have been described (
Dupuis et al. 2001,
2003;
Chapgier et al. 2006a,
2006b). Patients with complete loss-of-function of the STAT1 protein usually die in childhood from overwhelming viral and mycobacterial infections (
Dupuis et al. 2003). Other patients, who have the autosomal dominant
STAT1 loss-of-function form, usually present with a predisposition to low pathogenic mycobacterial infections (
Tsumura et al. 2012). Different mutations, mostly in the coiled–coiled domain, are believed to lead to gain of function of the STAT1 protein and cause an autosomal dominant form of chronic mucocutaneous candidiasis (CMC) (
Liu et al. 2011;
van de Veerdonk et al. 2011). Recently, a severe and fatal form of combined immunodeficiency was found to be associated with a declining immunity during childhood, caused by predominantly de-novo mutations in the
STAT1 DNA-binding domain (DBD) (
Sharfe et al. 2014).
The genetic form usually presents in a young infant with parental consanguinity or a history of other affected family members. Patients in this group have a genetic defect in genes that are responsible for the cytotoxic function of T cells and NK cells. Most commonly, mutations in the gene
PFR1 that encodes for Perforin1, are detected in this group of patients; however, other genes, such as
UNC13D and
STX11 that encode for cytolytic granule trafficking and exocytosis may also be associated with the aberrant inflammation (
Jordan et al. 2011). Although some of the primary immunodeficiency disorders that are associated with HLH have abnormal cytotoxic function of T cells and NK cells (for example Chédiak–Higashi syndrome and Griscelli syndrome type 2) others such as Wiskott–Aldrich and DiGeorge syndrome do not, and the mechanism leading to HLH in these conditions is not fully understood (
Introne et al. 1999;
Ménasché et al. 2000;
Cesaro et al. 2003;
Pasic et al. 2003).
In contrast to the genetic form of HLH, the acquired forms seem not to be associated with a known genetic abnormality or immunodeficiency syndrome. As in some of the genetic forms, the pathogenesis is not completely understood, and it seems that the disease appears in association with infection, malignancy, rheumatic conditions, metabolic disorders, or as a consequence of drug toxicity (
Henter et al. 1991;
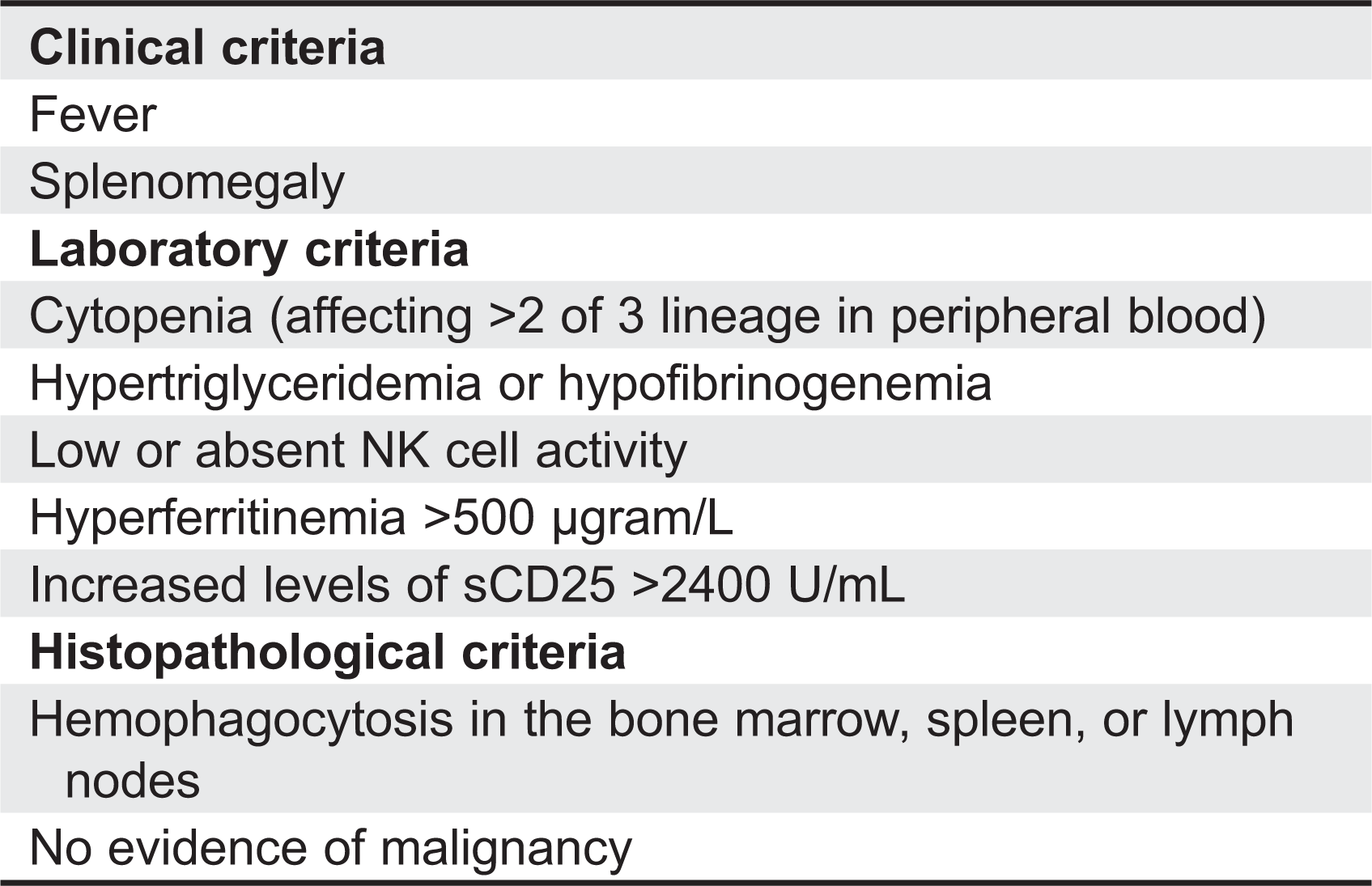
Janka et al. 1998). Nevertheless, it is conceivable that modifier genes may predispose individuals to developing secondary HLH. The symptoms and signs of HLH are nonspecific; fever, hepatosplenomegaly, lymphadenopathy, cytopenia, morbiliformic rash, and in some cases involvement of the central nervous system (CNS) with seizures or encephalopathy (
Henter et al. 2007).
Without treatment, the prognosis of HLH is poor, with less than 5% survival 1 year post diagnosis (
Janka 1983). Even with appropriate treatment, the mortality rate can be as high as 45% (
Henter et al. 2002). The main goal of the treatment is to suppress the exaggerated activation of the immune system with immunosuppressive agents such as dexamethasone, etoposide, and cyclosporine, and when needed and indicated a bone marrow transplantation (
Henter et al. 2002).
We report here an occurrence of fatal HLH in a patient with CMC due to STAT1 deficiency.
Methods
Flow cytometry
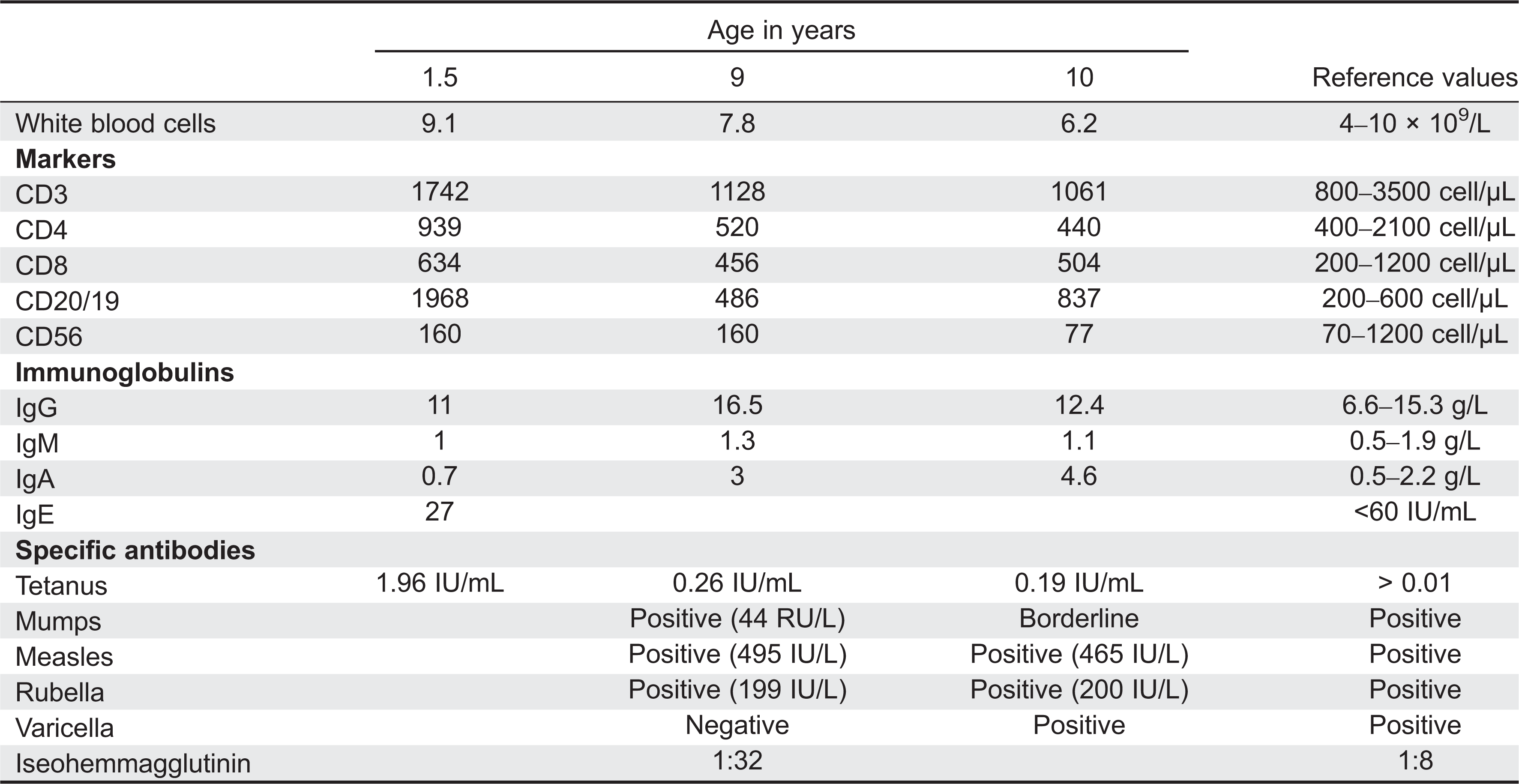
Peripheral blood mononuclear cells were obtained by Ficoll–Hypaque density gradient centrifugation, and surface phenotypes were determined by flow cytometry on a Coulter EPICS V flow cytometer (Beckman Coulter, Brea, Calif.), with a single argon laser, which analyzes up to 3 colours simultaneously. Single colour and (or) isotype antibody controls were both used for multicolour staining.
Proliferation assay
Lymphocyte proliferative responses to mitogens (including phytohemagglutinin and anti-CD3 antibodies) and to a panel of recall antigens (including Candida, tetanus, Herpes zoster, and cytomegalovirus) were determined by thymidine incorporation. All assays were performed in triplicate and were compared with simultaneously stimulated random normal controls.
DNA sequencing
Genomic DNA was extracted from peripheral blood lymphocytes using the Geneaid Genomic DNA Mini Kit. Genomic DNA was amplified by polymerase chain reaction (PCR) with specific primers for exons 1–23 of the coding sequence of STAT1 (NM_007315.3) (available upon request). Exons and their flanking intronic regions were sequenced with the GenomeLab Dye Terminator Cycle Sequencing Quick Start Kit (Beckman Coulter) and analyzed on a CEQ 8000 Genetic Analysis System (Beckman Coulter).
Discussion
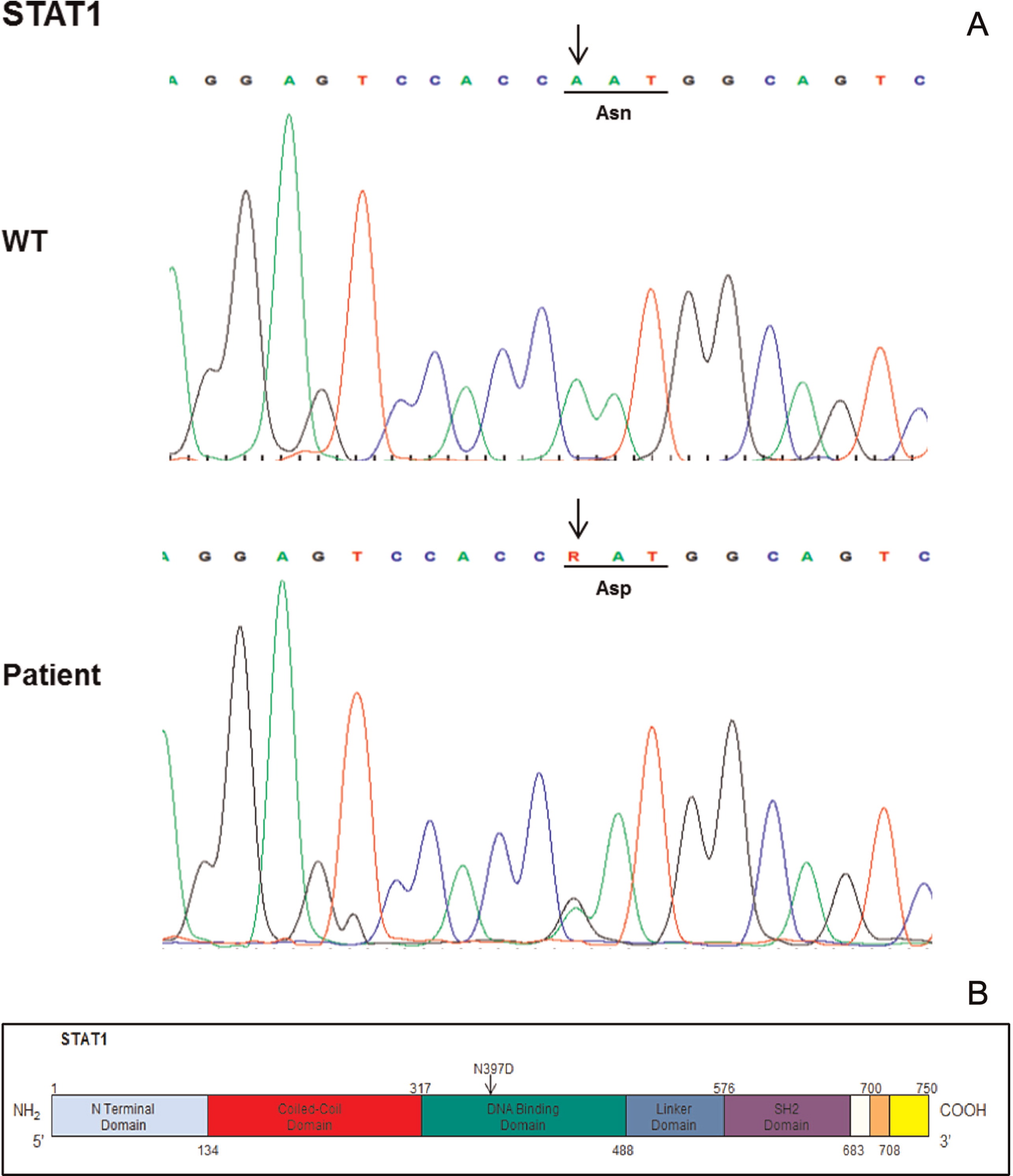
In the last few years, different mutations in the
STAT1 gene have been reported (
Liu et al. 2011;
van de Veerdonk et al. 2011;
Tsumura et al. 2012). Monoallelic mutations in the DBD of
STAT1 seem to cause a severe and frequently fatal immunodeficiency (
Sharfe et al. 2014). In this study we described a mutation in the DBD of the molecule. The fact that these mutations are de novo mutations, support the hypothesis that changes in the DBD of the
STAT1 gene are lethal at a young age before the carrier has a chance to produce offspring. The patient we described here had clinical symptoms of CMC and declining immunity that specifically displayed a decline in CD4
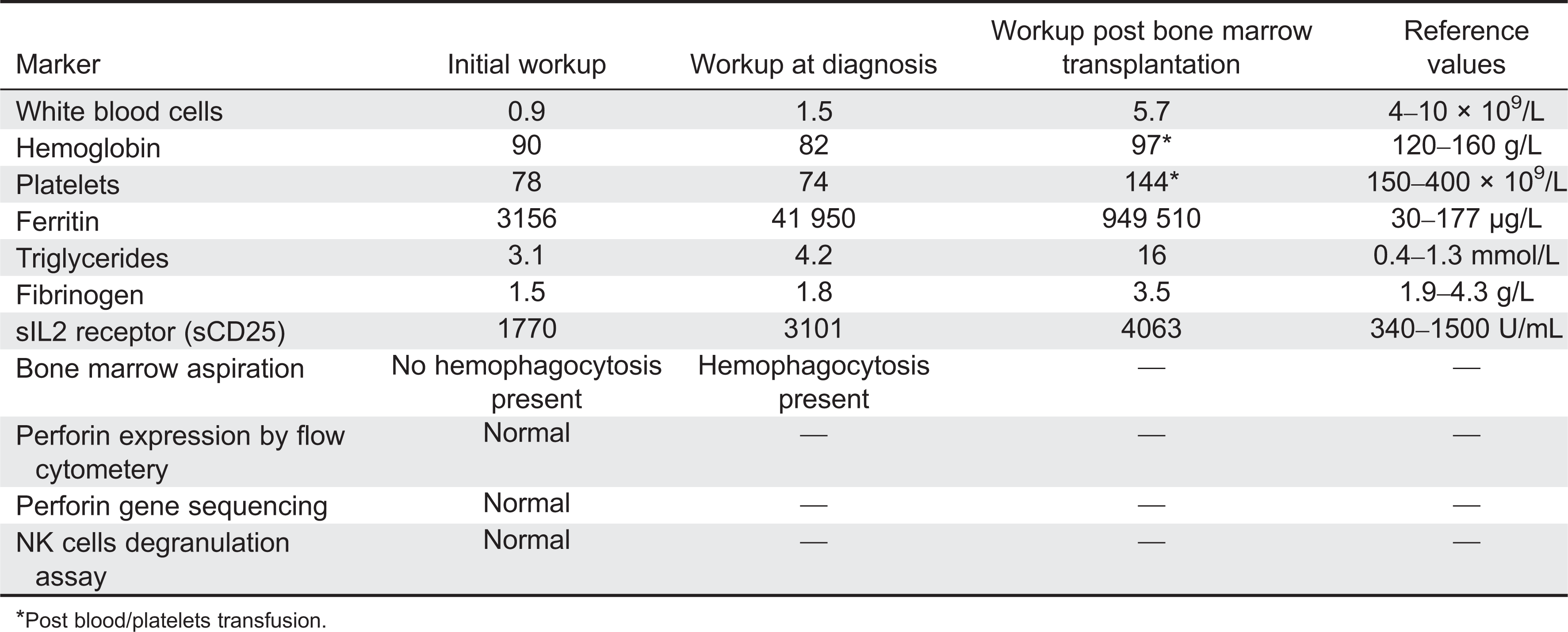
+ T cells and NK cells. The diminished number and function of NK cells were previously reported to be associated with HLH. In this case, it is also conceivable that the decline in NK cell numbers underscored at least in part the development of HLH, although the NK cell degranulation assay was normal.
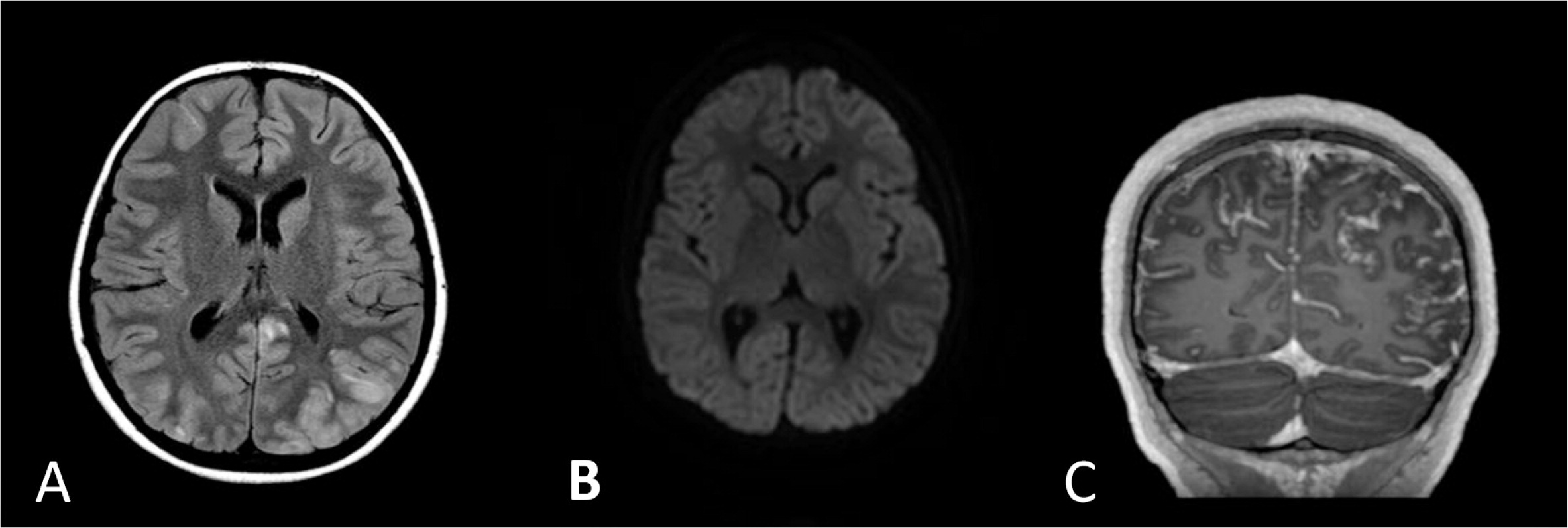
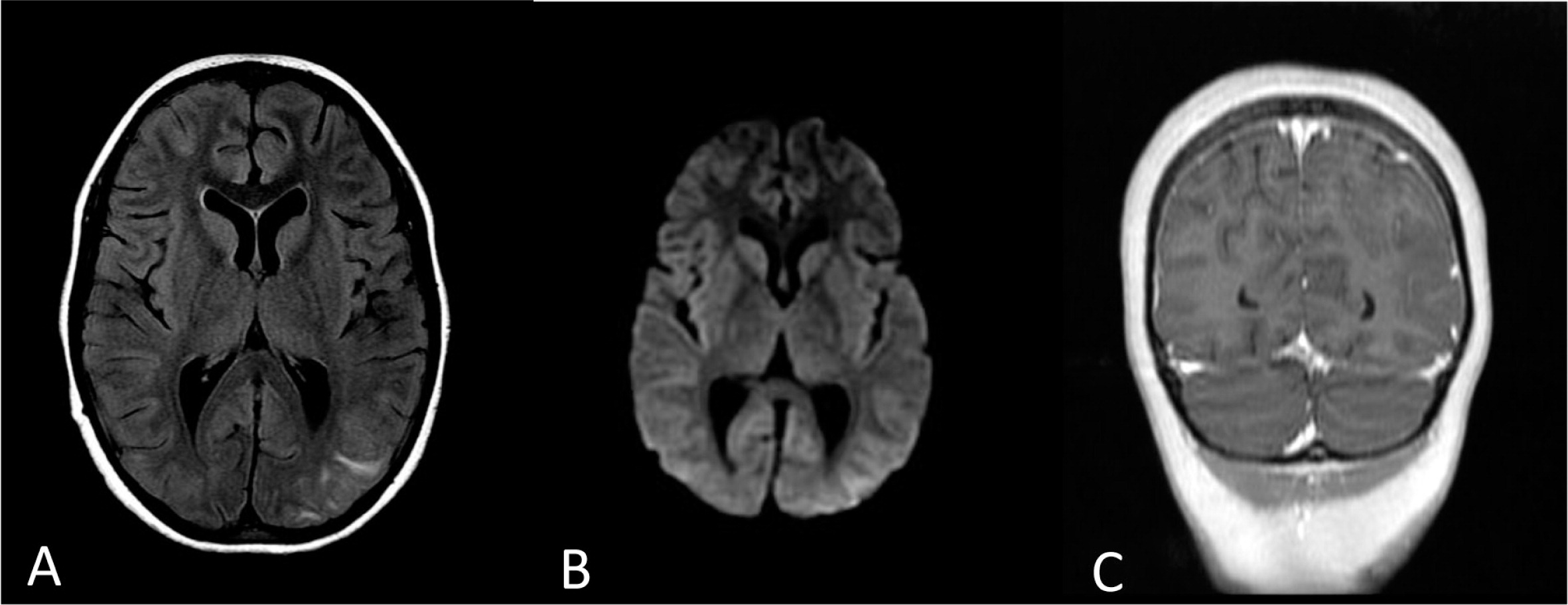
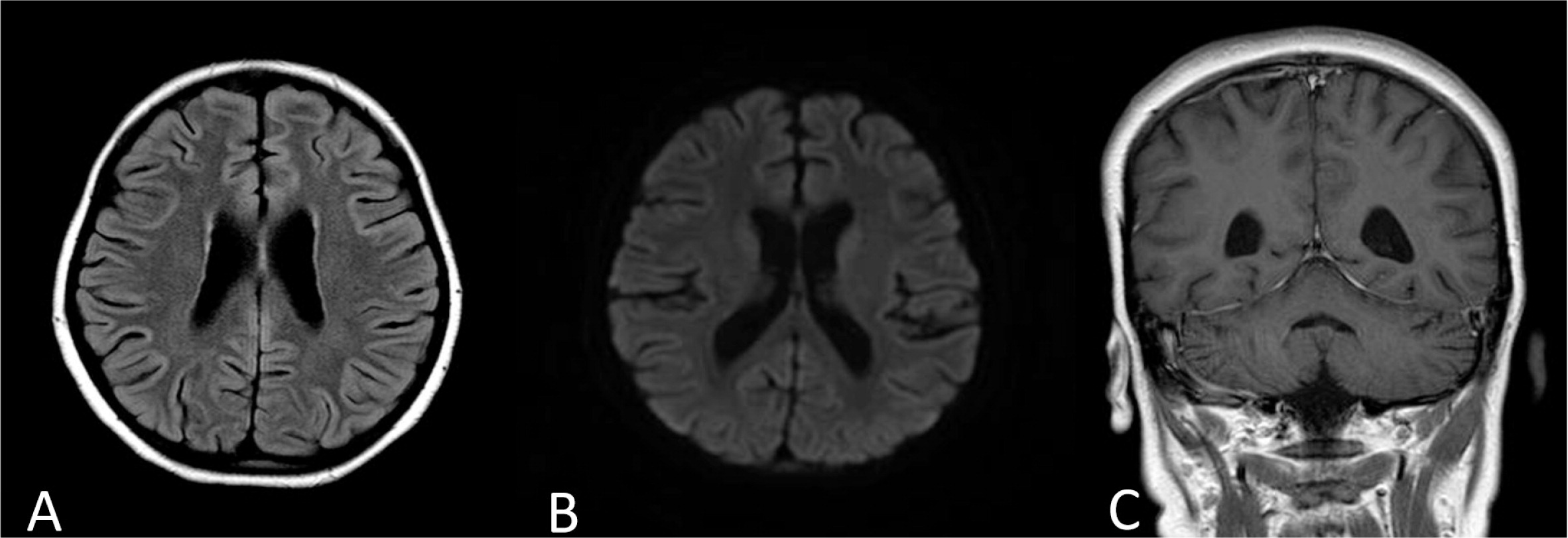
CNS involvement is common in HLH patients, and symptoms can range from abnormal findings in the cerebrospinal fluid to altered mental status, seizures, and coma. Active HLH, infection, and posterior reversible encephalopathy syndrome (PRES) are in the differential diagnosis in HLH patients with CNS involvement (
Haddad et al. 1997;
Goo and Weon 2007;
Horne et al. 2008;
Lee et al. 2013).
The overall survival of children with HLH who were treated with the HLH-94 protocol is around 55% and two-thirds of those who survived underwent BMT. It appears that survival after BMT in HLH cases is fair, regardless of whether a HLA matched sibling or an unrelated match donor are used (
Henter et al. 2007). Although there are some reports that support reduced intensity over full myeloablative conditioning (
Cooper et al. 2006;
Marsh et al. 2010), at present there is insufficient evidence to support the use of one protocol over another. Out of the few reports of BMT in patients with
STAT1 mutation, only 2 patients have been reported with long-term survival after receiving a BMT from a match sibling donor (
Deeg et al. 1986;
Hoh et al. 1996), whereas 2 other patients died after the procedure (
Aldave et al. 2013).
In conclusion, we have shown an association between a monoallelic STAT1 mutation and a severe and refractory case of HLH. A thorough immunologic workup that includes STAT1 genetic analysis is needed in severe and refractory cases of HLH to identify those who have this uncommon genetic abnormality. More studies are in order to define optimal treatment options for patients with STAT1 mutation and HLH.