Case series of COVID-19 outcomes in adult patients with inborn errors of immunity
Abstract
Introduction
Methods
Results
Patient characteristics
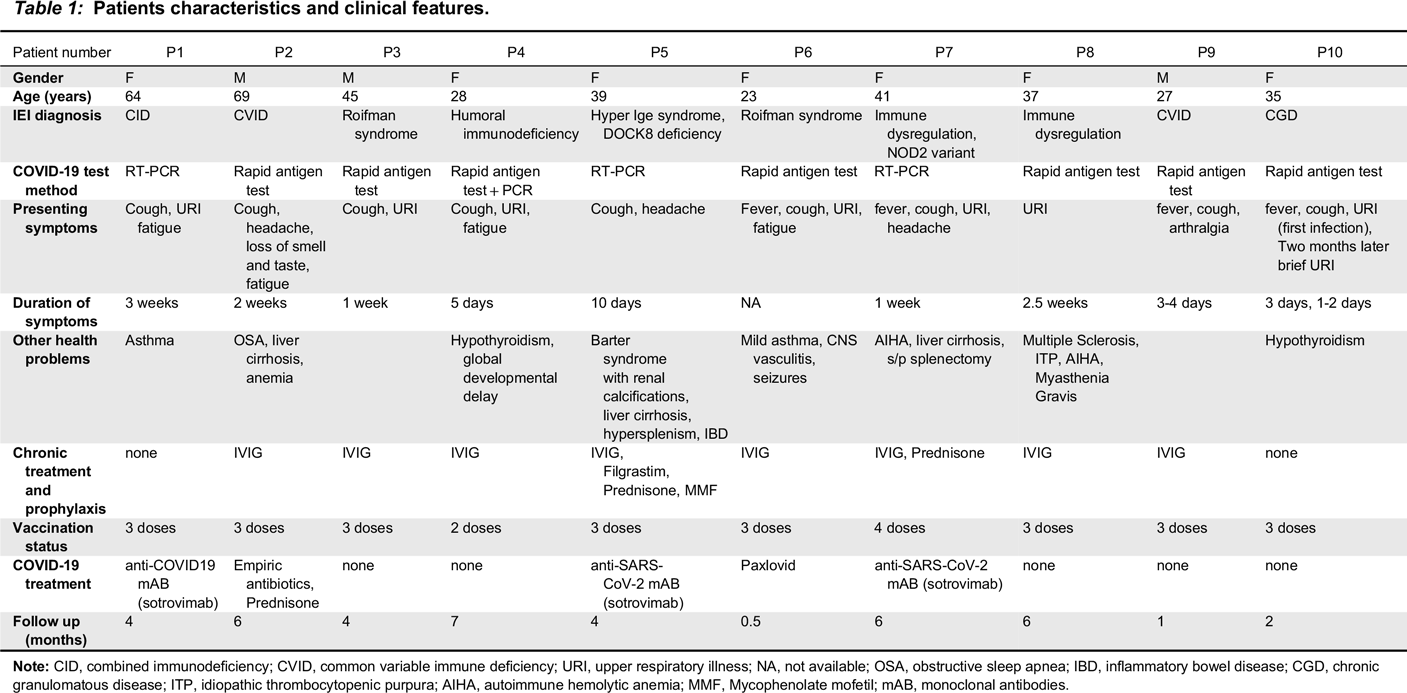
Note: CID, combined immunodeficiency; CVID, common variable immune deficiency; URI, upper respiratory illness; NA, not available; OSA, obstructive sleep apnea; IBD, inflammatory bowel disease; CGD, chronic granulomatous disease; ITP, idiopathic thrombocytopenic purpura; AIHA, autoimmune hemolytic anemia; MMF, Mycophenolate mofetil; mAB, monoclonal antibodies.
Clinical course, immunoglobulin replacement, and vaccination
COVID-19 treatments
Follow up
Discussion
REFERENCES
Information & Authors
Information
Published In

History
Copyright
Authors
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
There are no citations for this item
View Options
View options
Login options
Check if you access through your login credentials or your institution to get full access on this article.


