Background
Coronavirus disease 2019 (COVID-19) is a rapidly spreading pandemic caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since its origins in Wuhan, Hubei Province of China, in December 2019, the disease has affected more than 100 million people worldwide, and with over 2 million deaths as of February 2021 according to the World Health Organization (WHO) COVID-19 Dashboard (
Organisation 2021). As of March 2021, COVID-19 has resulted in over 6,000 deaths in Israel, while more than 500 patients are currently in a severe condition, including 250 whom are mechanically ventilated (
Datadashboard.health.gov.il n.d).
The impact of SARS-CoV-2 infections in children has generally been described as relatively benign (
Castagnoli et al. 2020). However, since April 2020, there have been reports of a new multisystem inflammatory illness affecting children, related to COVID-19, termed multisystem inflammatory syndrome in children (MIS-C), sometimes also described as “pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2” (PIMS) (
Ng et al. 2020;
Riphagen et al. 2020;
Verdoni et al. 2020;
Viner and Whittaker 2020). Patients with MIS-C exhibit similar symptoms to those found in Kawasaki disease (KD), and streptococcal and staphylococcal toxic shock syndromes (TSS); however, there are several key clinical, epidemiological, and importantly immunological features that are unique to this syndrome (
RCPCH n.d;
Arad et al. 2011;
Abrams et al. 2020;
Ahmed et al. 2020;
Consiglio et al. 2020). Here, we describe 3 cases of this newly described disease and discuss its spectrum.
Case presentations
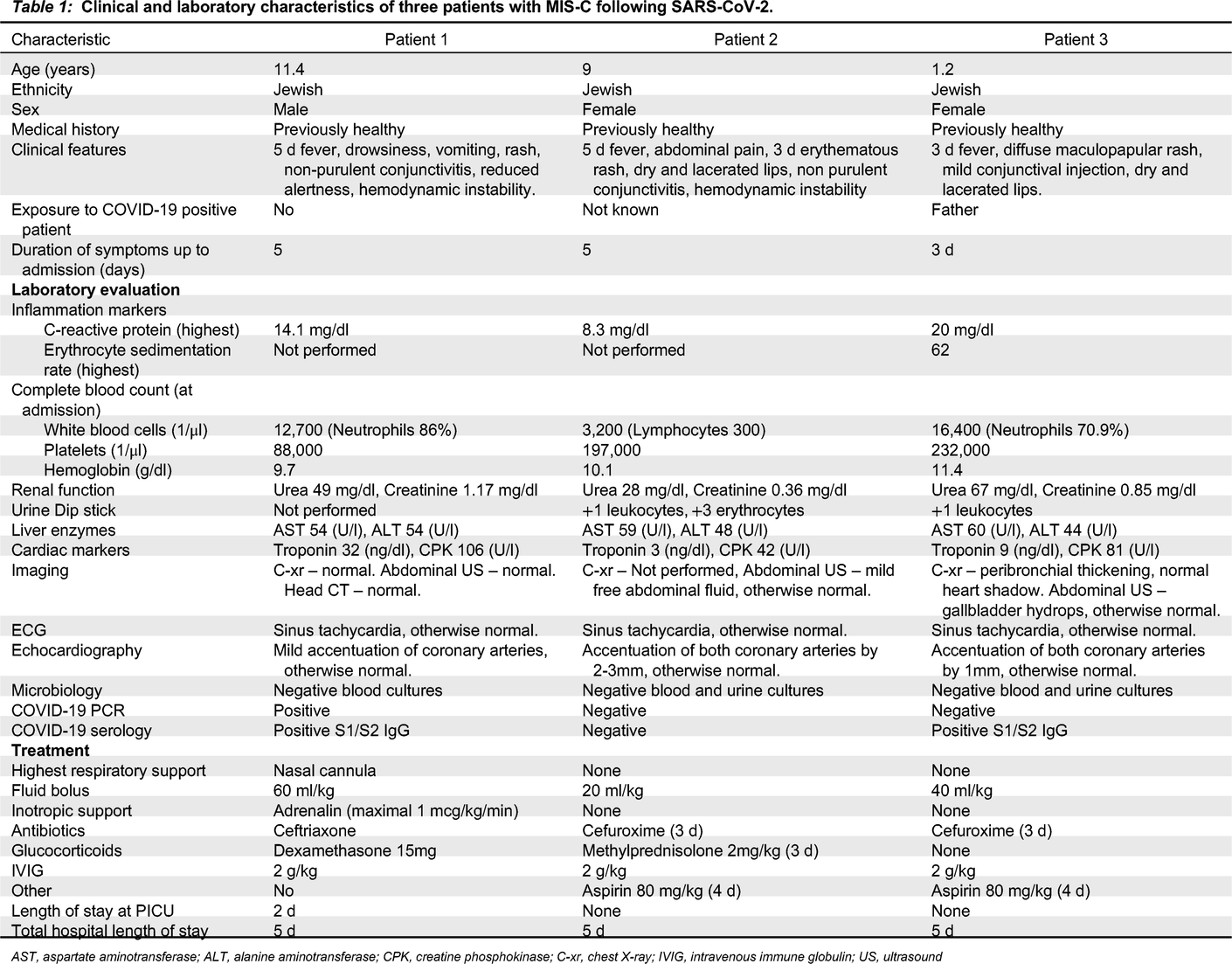
All patients described below were admitted to our pediatric ward between October 2020 and January 2021. All clinical and laboratory manifestations are summarized in
Table 1.
Patient 1, an 11-year-old male, presented in the pediatric emergency room with a medical history of 5 days fever and vomiting, new onset of drowsiness, diffuse maculopapular rash, non–purulent conjunctivitis, and cracked red lips. On physical examination, he was found to have meningeal irritation and nuchal rigidity. He was treated with a fluid bolus and antibiotics on a working diagnosis of bacterial meningitis, but within an hour he deteriorated and developed hemodynamic instability leading to cardiovascular shock that required vasopressor support.
Patient 2, a 9-year-old female, presented with abdominal pain. She was suspected to have appendicitis based on the clinical picture, and was hospitalized for further investigation. While hospitalized, she developed KD-like symptoms including fever, rash, cracked lips, and non-purulent conjunctivitis. In addition, she also developed low blood pressure that responded well to fluid treatment.
Patient 3, a 1-year-old female, presented with a 3 day fever, and later developed a rash, mild conjunctival injection, and cracked lips.
All patients had strikingly elevated inflammation markers. Other hematological abnormalities are presented in
Table 1. Two patients (1 and 3), had a transient acute kidney injury, while all 3 had a mild elevation of liver enzymes that later resolved. Patient 1 had a mildly elevated troponin I, while patient 2 had sterile pyuria on admission. Chest X-ray was performed for all patients with no significant findings. Patient 2 had low amounts of free abdominal fluid on ultrasonography; patients 3 had hydrops of the gallbladder. Electrocardiography was normal except for sinus tachycardia in all patients. Echocardiography demonstrated prominent coronary arteries for all patients, cardiac function was normal; patient 1 had a mild pericardial effusion.
All patients underwent extensive microbiological investigation (
Table 1).
SARS-CoV-2 polymerase chain reaction (PCR) testing was positive for patient 1, while Anti-SARS-CoV-2 immunoglobulin G (IgG) was positive for patients 1 and 3. The serology testing was performed using the DiaSorin (Saluggia VC, Italy) Liaison SARS-CoV-2 S1/S2 IgG assay, which detects antibodies specific to the SARS-CoV-2 spike (S) proteins. All other infectious investigations were negative.
All patients were treated with a fluid bolus and intravenous immunoglobulin (IVIG). Treatment with wide spectrum antibiotics was initiated in all 3 patients until negative results of blood and urine cultures. Patients 1 and 2 were also treated with glucocorticoids. Patient 1 was admitted to the pediatric intensive care unit (PICU) for vasopressor (adrenaline) and respiratory support (nasal cannula) for 1 day, the patient responded well to treatment with a rapid clinical improvement and was discharged from the PICU to the pediatric ward after 2 days. All 3 patients were discharged home after 5 days.
Discussion
Accumulating evidence that an inflammatory syndrome may follow SARS-CoV-2 infection in some children is in contrast to the general impression that COVID-19 is mostly asymptomatic in children but may present with mild respiratory or gastrointestinal symptoms (
Castagnoli et al. 2020).
The Royal College of Pediatrics and Child Health (RCPCH), center for disease control (CDC) and WHO have fairly similar but not identical criteria for the emerging condition of MIS-C. All 3 cite inflammation, and single or multi organ dysfunction, although the RCPCH does not require virological evidence, while the WHO and the CDC criteria include viral positive PCR or serology, or close contact to a known COVID-19 patient (
RCPCH n.d.;
www.who.int n.d.;
Centers for Disease Control and Prevention 2021;
Riphagen et al. 2020). All 3 cases in our report fulfilled the RCPCH case definition, while patients 1 and 3 also satisfy the CDC and WHO criteria. Patient 2 had no virological evidence of infection; however, the presentation is highly suggestive of the disease (
RCPCH n.d;
www.who.int n.d.;
Centers for Disease Control and Prevention 2021).
All our patients had coronary changes when diagnosed, and while coronary findings are well described in MIS-C (
Alsaied et al. 2021), the incidence varies significantly among reports; larger series have reported coronary abnormalities in 8-24% of cases (
Valverde et al. 2021).
Although similar, KD and MIS-C have important differences in phenotype and laboratory profile. MIS-C tends to manifest in older children. Patients have more gastrointestinal involvement and are more prone to severe hemodynamic involvement including shock. While KD is known to cause thrombocytosis, MIS-C patients have variable platelet counts, other laboratory findings specific to MIS-C include lymphopenia and elevated ferritin (
Chen et al. 2021). Another important difference is disease prognosis; while up to 5% of adequately treated patients with KD might still have significant coronary changes, the prognosis for MIS-C seems to be excellent (
Eleftheriou et al. 2013;
Valverde et al. 2021).
The association between MIS-C and SARS-CoV-2 infection was suggested by the temporal relation and clustering of cases with the rise of the pandemic (
European Centre for Disease Prevention and Control 2020). An increasing number of studies reported high rates of serologic positivity to SARS-CoV-2: a UK case series found 85% IgG positivity (
European Centre for Disease Prevention and Control 2020); a study from Italy describing ten patients found similar IgG positivity rates (
Verdoni et al. 2020); finally a French study reported that 90% of their 21 patients had anti-SARS-CoV-2 IgG (
Toubiana et al. 2020). This might suggest a causative and perhaps immunologically mediated relation between SARS-CoV-2 infection and seroconversion in this syndrome.
Studies attempting to explain this relationship have shed light on the basic molecular biology processes that might explain this syndrome. One study by Rivas et al. (2021) noted that the SARS-CoV-2 spike protein encodes a high-affinity SAg-like sequence motif near the S1/S2 cleavage site of the spike protein, which exhibits a high affinity for T-cell receptors (
Noval Rivsa et al. 2020). Interestingly, the region is very similar in sequence and structure to a fragment of the super-antigenic Staphylococcal Enterotoxin B (SEB) that is known to cause the cytokine storm typical of TSS (
Arad et al. 2011;
Cheng et al. 2020).
A study by Consiglio et al., published in the journal Cell in November 2020, described multiple aspects of the hyper-inflammatory response in children with MIS-C. The study revealed some similarities with KD but also demonstrated important differences, of which one such example is the lack of interleukin-17 (IL-17) mediated hyper-inflammation in MIS-C. Other differences might include changes in T cell populations suggesting immune dysregulation (
Consiglio et al. 2020).
This syndrome is one of the multiple long term effects of SARS-CoV-2. Other long term effects have been described by a number of studies from different countries, the largest being from China (
Xiong et al. 2021), and the US (
Taquet et al. 2020). A systematic review of studies from many countries with follow-up of up to 110 days found that 80% of patients had one or more symptoms on long term follow-up, with the most common being fatigue, headache, attention disorder, and dyspnea. These studies included only adult patients (
Lopez-Leon et al. 2021). Data for the pediatric population is scarce (
Ludvigsson 2021).
In summary, we present 3 cases of MIS-C and discuss its spectrum. The short term and long-term effects of this entity require further investigations.