Introduction
On 31 January, 2020, the U.S. Department of Health & Human Services (HHS) Secretary declared a public health emergency related to the virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), that causes coronavirus disease 2019 (Covid-19) and was followed by the World Health Organization’s (WHO) declaration of a global Covid-19 pandemic. Up to 29 March, 2021, SARS-CoV2 has infected over 125 million people worldwide and claimed more than 2.8 million human lives (
Lamb 2020;
Lewis 2020;
US Department of Health Human Services 2020;
WHO 2020;
Our World in Data 2021).
Following HHS’ announcement, the US Food and Drug Administration (FDA) issued immediate, in effect guidance on 29 February, 2020, related to the development of in vitro diagnostic tests during this public health emergency. Shortly after FDA’s announcement, hundreds of manufacturers developed a variety of diagnostic and serological in vitro tests and began providing these kits to health care providers and laboratories. Testing for SARS-CoV-2 can be performed by either testing for the virus (diagnostic) or testing for past infection by assessing antibody response produced by the host (serological) (
CDC 2020a). Since the Covid-19 pandemic started, reverse-transcriptase polymerase chain reaction (RT-PCR) tests have been the mainstay of diagnosis. However, the supply and demand for these diagnostic tests has been variable depending on waves of infections occurring at different locations. The Covid-19 RT-PCR usually involves upper or lower respiratory specimens as test samples. Upper respiratory specimens include nasopharyngeal, oropharyngeal, or nares swabs. Results can be expected within a few hours to days depending on laboratory capacity and volume of samples to be tested. The sensitivity of RT-PCR testing varies depending on timing of specimen collection in relation to days following symptoms, viral inoculum, sample collection site, and the assay’s limit of detection. Studies have documented a sensitivity range between 50%–80% (
Basu et al. 2020;
Bhimraj et al. 2020;
Guo et al. 2020;
Hanson et al. 2020;
Vabret 2020;
Zhao et al. 2020).
In the United States, the current shortage of reagents to run tests has resulted in longer turnaround times and test rationing. Fifteen (15) minute rapid lateral flow immunoassay (LFI) antibody tests can be used to quickly assess past infection as described in prior studies (
CDC 2020c,
Yang et al. 2020). Anti-SARS-CoV-2 antibodies typically become detectable starting approximately 1 week after onset of symptoms, with IgM antibodies detectable around day 5–10 after onset of symptoms and IgG antibody levels following the IgM response soon thereafter (
Deeks et al. 2020;
Guo et al. 2020). Serologic tests, therefore, are not useful early in the course of illness for diagnosing Covid-19. Furthermore, not all patients with SARS-CoV-2 infection develop an antibody response, and so a negative serologic result does not exclude past infection. The aim of this study was to understand seroconversion in patients diagnosed with Covid-19 in relation to their symptoms using a rapid 15-minute Point-Of-Care test (Clungene
® lateral flow immunoassay (LFI), and the positive or negative percentage agreement with RT-PCR testing.
Study design
This was a formal IRB approved clinical study conducted within Sharp Healthcare, a not-for-profit multi-center regional health care group located in San Diego, California. Subjects were included if hospitalized or recently discharged following a SARS-CoV-2 RT-PCR nares test. The Cobas® Roche platform was used for detection of SARS-CoV-2 RNA and performed at the Sharp Healthcare Laboratory. A study protocol and an informed consent were initiated and approved by the Sharp Institutional Review Board. Subjects were included if >18 years of age and understood the study and its requirements. Patients who had impairment of cognition or decision-making capacity were excluded. Subjects were screened by research coordinators to determine if they had a nares SARS-CoV-2 RT-PCR test result and then consent was requested to enroll in the study.
There were three (3) groups of subjects. Arm 1: Subjects with positive a SARS-CoV-2 RT-PCR positive test result were serologically tested twice with the Clungene® immunoassay; the first test was performed up to 14 days following self-reported onset of symptoms and the second between day 12 and 70. After discharge, subjects were contacted by phone and requested to come into the clinic to have a finger prick for the blood collection. If patients were unable to come to the clinic, study staff took blood samples at home. Arm 2: A second cohort of hospitalized patients with a positive SARS-CoV-2 RT-PCR test result were serologically tested once with the Clungene® immunoassay 12–70 days after symptoms onset. Arm 3: Patients with a negative SARS-CoV-2 RT-PCR test result were serologically tested once between 1 and 10 days with the Clungene® immunoassay following a negative RT-PCR test result.
Methods
The Clungene® Point-Of-Care test was run according to manufacturer’s instructions. The test was performed by three (3) independent study coordinators who confirmed that the test was properly working and simple to operate. The test result was read after 15 minutes. Leftover blood was used in the inpatient setting; whole blood finger prick samples were used at the outpatient clinic after discharge.
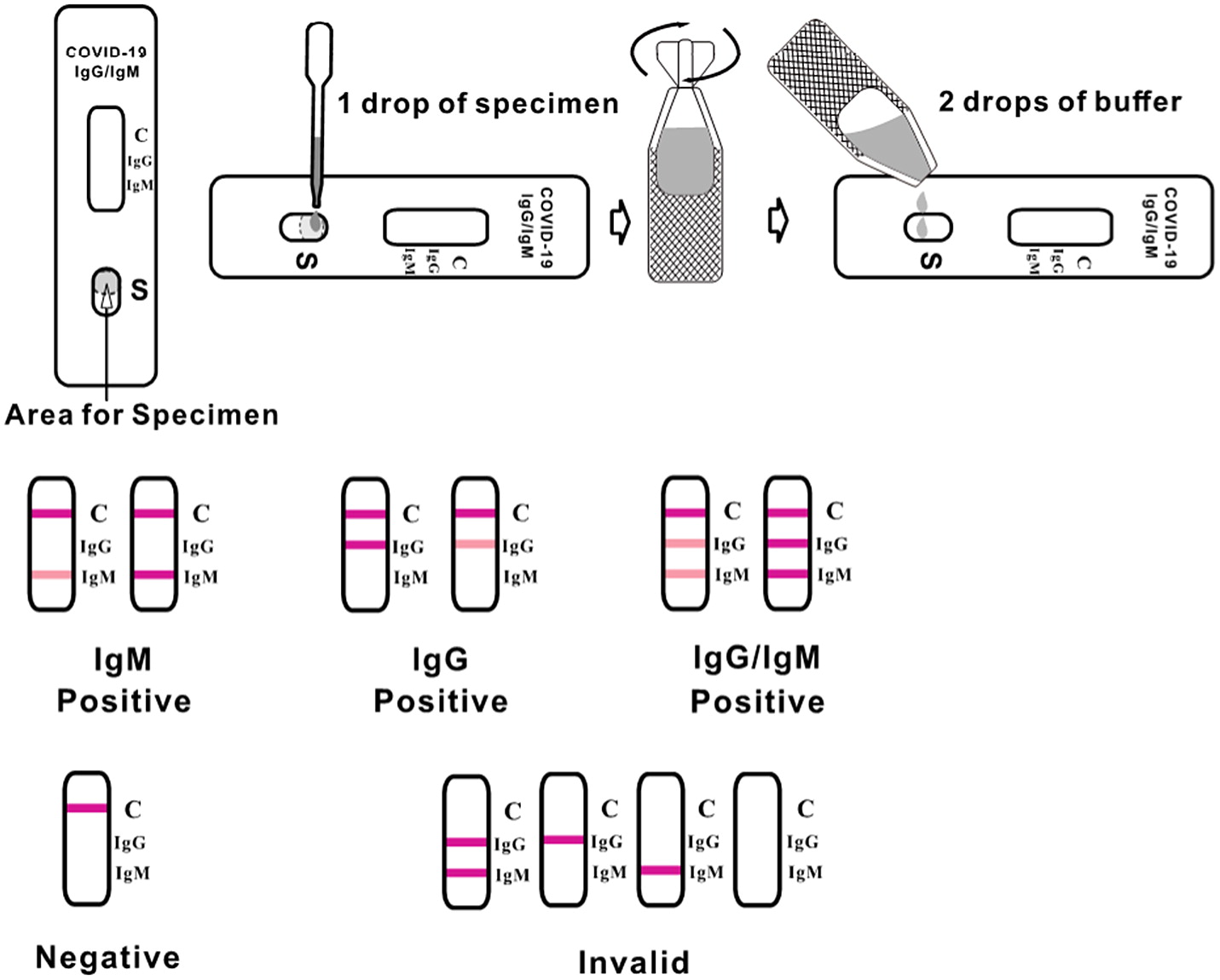
The Clungene
® SARS-COV-2 virus (Covid-19) IgG/IgM rapid test cassette is a qualitative membrane strip-based immunoassay for the detection of antibodies (IgG and IgM) to SARS-CoV-2 in human whole blood, serum, or plasma. The test cassette consists of a burgundy-colored conjugate pad containing SARS-CoV-2 virus recombinant envelope antigens conjugated with colloid gold (SARS-CoV-2 conjugates). It also has a nitrocellulose membrane strip containing 2 test lines (IgG and IgM lines) and a control line (C line). The IgM line is pre-coated with the Mouse anti-Human IgM antibody, IgG line is coated with Mouse anti-Human IgG antibody. The test principle is based on the receptor-binding domain (RBD) of the spike and nucleocapsid proteins. The serum level of RBD-binding antibodies correlates with SARS-CoV-2 neutralization. Previous studies have shown that the Clungene
® performs well when evaluating convalescent plasma donors and patients presenting at physicians’ offices (
Osher et al. 2020;
Ransegnola et al. 2020).
When an adequate volume of test specimen is dispensed into the sample well of the test cassette, the specimen migrates by capillary action across the cassette. IgM anti-SARS-CoV-2, if present in the specimen, will bind to the SARS-CoV-2 conjugates (
Our World in Data 2021). The immunocomplex is then captured by the reagent pre-coated on the IgM band, forming a burgundy-colored IgM line, indicating a SARS-CoV-2 IgM positive test result. IgG anti-SARS-CoV-2 if present in the specimen will bind to the SARS-CoV-2 conjugates. The immunocomplex is then captured by the reagent coated on the IgG line, forming a burgundy-colored IgG line, indicating a SARS-CoV-2 IgG positive test result. Absence of any T lines (IgG and IgM) suggests a negative result. To serve as a procedural control, a colored line will always appear at the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred. See
Figure 1.
Days from symptom onset were captured from the electronic medical record (EMR) which documented self-reported data from patients on the number of days they had been sick at the time of study enrollment. Symptoms of Covid-19 included fever, weakness, cough, shortness of breath, tiredness, anosmia, and loss of taste. Patient characteristics were extracted from the EMR by clinical research coordinators who documented age, gender, body mass index (BMI), comorbid conditions, maximum temperature, C-reactive protein (CRP), and ferritin levels at time of enrollment.
Statistical analysis
Categorical variables were compared using the chi-squared or Fisher exact test, and continuous variables were compared using the Student t-test or Mann-Whitney U test, as appropriate. All tests were 2 tailed, and P < 0.05 was considered statistically significant. SPSS Statistics, IBM SPSS software, version 27.0 (SPSS, Inc., Chicago, IL) was used for all calculations.
Results
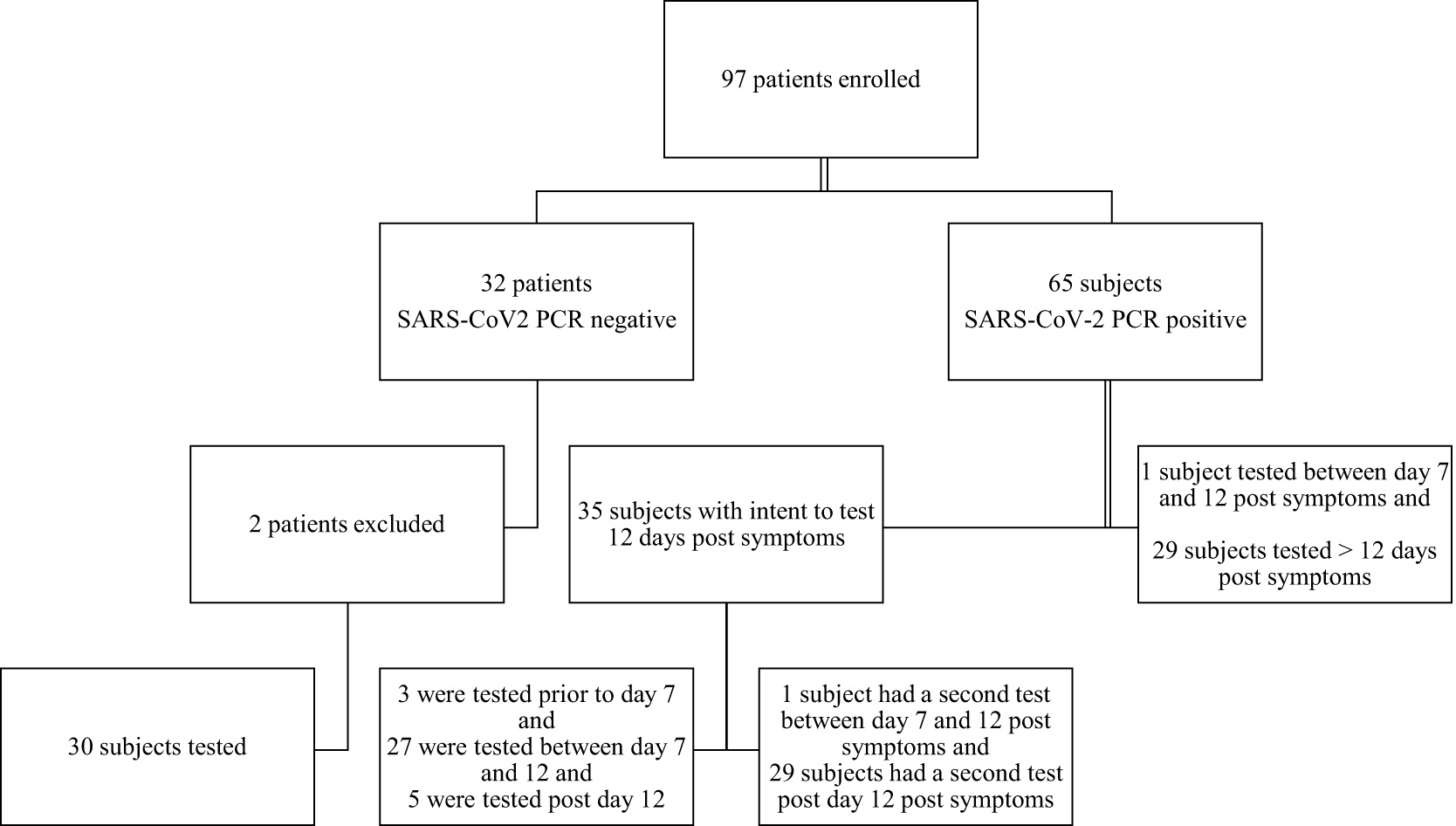
Between May 2020 and August 2020, 97 subjects were enrolled, consented, and tested. Two potential subjects who tested negative for SARS-CoV2 RT-PCR tests were excluded by the principal investigator. After enrollment, it was learned that 1 patient had a pre-hospital positive SARS-CoV-2 RT-PCR test. The second patient was excluded due to 30 days having lapsed between the negative RT-PCR test and the antibody test in a high-risk hospital environment.
Figure 2 displays a schematic of patients included in the final study sample size.
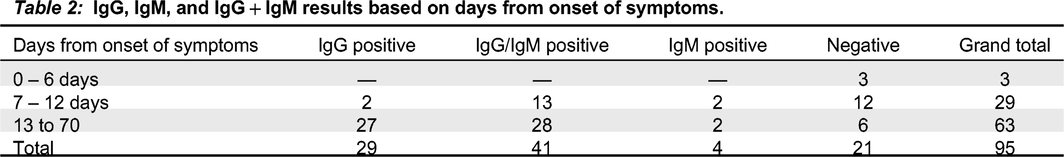
A protocol amendment allowed for patients to be tested in the outpatient setting post discharge after 14 days from symptom onset. An analysis was run on 95 patients who completed the study. Thirty patients who had negative Covid-19 RT-PCR were tested and found antibody negative using the Clungene
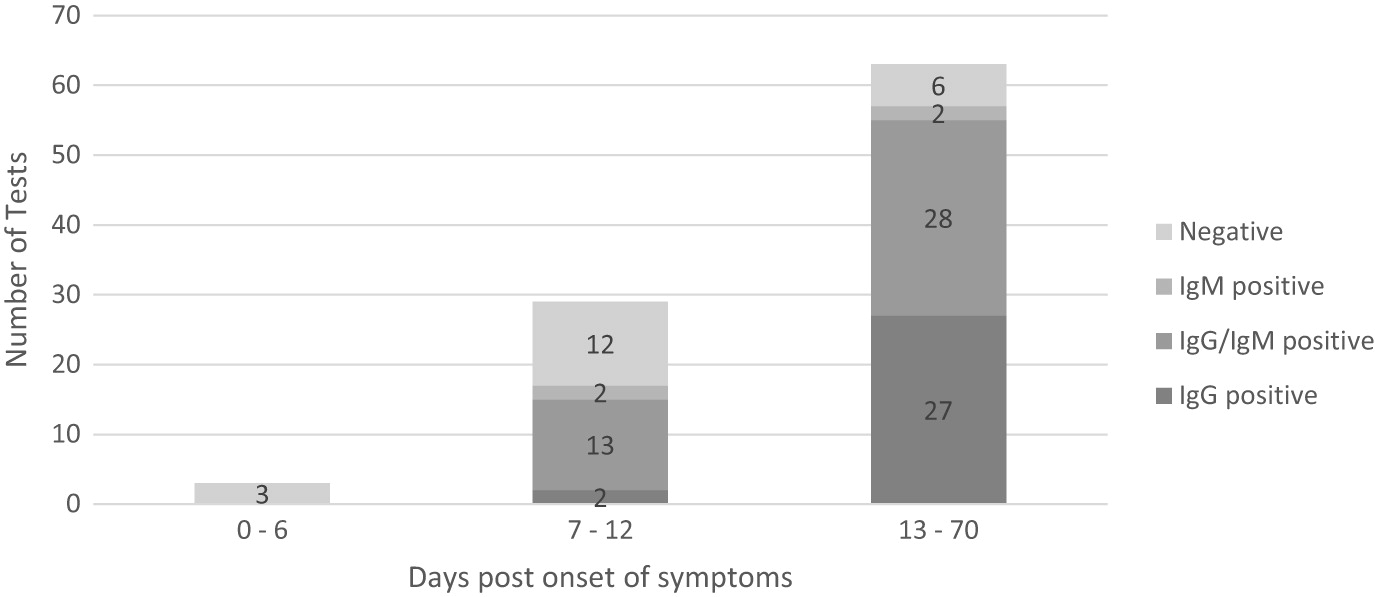
® test. Three (3) RT-PCR positive Covid-19 subjects tested prior to day 7 symptom onset were antibody negative. 29 subjects were tested between day 7 and 12 from symptoms and 63 were tested after day 12 from symptom onset. In patients with confirmed Covid-19 with RT-PCR, the combined sensitivity of IgM and IgG was 58.6% (95% CI, 38.9%–76.5%) between day 7 and day 12 from symptom onset and 90.5% (95% CI, 80.4%–96.4%) after day 12. The specificity was 100% (95% CI, 88.4-100.0%), meaning there was 100% agreement between a negative RT-PCR test and 100% negative antibody result. These results are displayed in
Table 2.
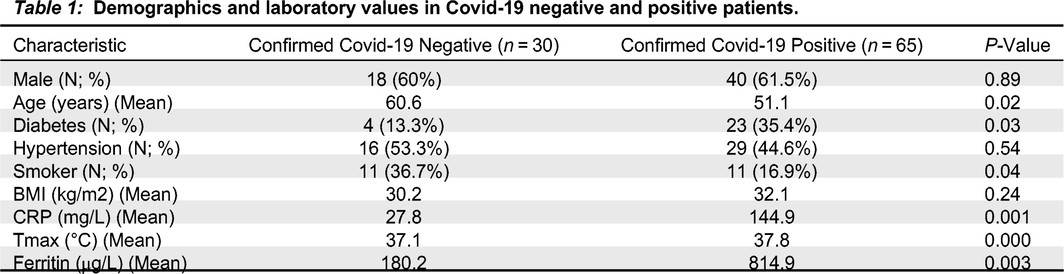
Overall, the median (IQR) days to RT-PCR testing from symptom onset was 7.5 days (IQR, 4-10 days). Compared to subjects with negative Covid-19 RT-PCR tests (
n = 30), patients with Covid-19 (
n = 65) were younger (51.1 years vs. 60.6 years,
P = 0.02), and had higher CRP (144.9 mg/L vs. 27.8 mg/L;
P = 0.001), ferritin (814.9 μg/L vs. 180.2 μg/L;
P = 0.003), and Tmax (37.8 °C vs. 37.1 °C;
P < 0.001) values at time of enrollment (
Table 1).
There was a statistical difference in sampling time to the onset of symptoms and true positive results (11.4 days vs. 22.3 days;
P < 0.001), with samples collected after a longer period upon symptom onset associated with higher sensitivity. In the 15 patients with false negative results prior to 14 days from symptom onset, 13 patients seroconverted in a median 11 days (7.5–36.0 days) (8 IgG, 1 IgM, 4 IgG + IgM) post median 9 days (7.5–10.5 days). Results of the test according to the number of days from the onset of symptoms are presented in
Figure 3.
Smoking was significantly less prevalent in the positive group as presented in
Table 1. This was an unexpected result since tobacco smokers are thought to be more vulnerable to contracting Covid-19, as the act of smoking involves contact of fingers (and possibly contaminated cigarettes) with the lips, which increases the possibility of transmission of viruses from hand to mouth. However, we are not aware of any peer-reviewed studies that have evaluated the risk of SARS-CoV-2 infection associated with smoking. Given the small sample size, this may be an anomaly and should be investigated further.
Discussion
In this study, the performance characteristics of Clungene
® were evaluated and showed a specificity of 100% and a sensitivity of 90.5% for samples collected more than 12 days after the onset of symptoms. These results are consistent with previously reported results (
Flower et al. 2020;
Nicol et al. 2020;
Wu et al. 2020;
Zhu et al. 2020). Since March 2020, testing for Covid-19 in the United States has undergone multiple phases. From the U.S. Centers for Disease Control and Prevention (CDC) to U.S. individual state health departments, the availability for testing has remained constrained. There are certain limitations that make RT-PCR testing less readily available: shortage of RT-PCR kits, swabs, and chemical reagents. The requirement for trained laboratory personnel and the lack of protective personal equipment to collect RT-PCR samples also plays a factor. Additionally, the number of available public labs (784 in the U.S.), and their centralization, has led to RT-PCR results taking days and even weeks (
CDC 2020b).
Antibody testing can provide a useful aid for diagnosis. Recently, the Infectious Disease Society of America (IDSA) updated their serology Covid-19 recommendations to include testing in certain limited circumstances (
CDC 2020a). In addition to the use of antibody testing in epidemiologic prevalence studies, the CDC recommends antibody testing for persons suspected of having a post-infectious syndrome caused by Covid-19 (e.g., Multisystem Inflammatory Syndrome in Children) (
CDC 2020a). Sharp Healthcare recommends antibody testing in the latter situation and if 2 consecutive RT-PCR tests are thought to be false negatives. This guidance discourages clinicians from using antibody testing for diagnosis and a recommendation was made to use these tests as complimentary to RT-PCR testing. We see the potential for a much broader use and recommend a combined approach that adapts using both RT-PCR and serological testing especially after the first week of illness. The advantage of the Clungene
® Point-Of-Care antibody test is its simplicity since there is no need for laboratory personnel to perform and interpret results. The low rate of false positivity makes this test ideal to rule in disease and eliminate the need for further RT-PCR testing if seroconversion occurs.
Preliminary studies proposed that IgM antibodies against SARS-CoV-2 may appear earlier than IgG, and that measuring both IgM and IgG concomitantly would improve the diagnosis of SARS-Cov-2 infection (
Basu et al. 2020;
Guo et al. 2020;
Vabret 2020;
Zhao et al. 2020). A recent Cochrane Review examining the diagnostic accuracy of antibody tests in 57 publications determined combination of IgG/IgM had a sensitivity of 30.1% (95% CI, 21.4–40.7%) for 1 to 7 days, 72.2% (95% CI, 63.5–79.5%) for 8 to 14 days, 91.4% (95% CI, 87.0–94.4%) for 15 to 21 days. They also concluded there is insufficient data to estimate the sensitivity of serology 35 days or more post-symptom onset (
Hanson et al. 2020). Our data supports the Cochrane review, and we see no diminution of antibody positive subjects post 35 days (100% positive in 10 subjects).
Additionally, Zhao et al found that in 173 patients with SARS-CoV-2 infection, confirmed by RT-PCR in the early phase of illness (within 7-day since onset), RT-PCR had the highest sensitivity of 66.7%, whereas the antibody assays had a positive rate of 38.3% (
CDC 2020c). However, the presence of antibodies increased to 100% (Ab), 94.3% (IgM) and 79.8% (IgG) 15 days after the onset of symptoms. Combining RT-PCR and antibody tests significantly improved the sensitivity of the diagnosis for Covid-19 (
P < 0.001), even in early phase of 1-week since onset (
P = 0.007). These findings suggest that serological testing can be a critical addition to RNA detection during the illness course.
Antibody testing for diagnosis in the first twelve (12) days of illness is not recommended since most patients develop antibodies later in the course of disease. However, if RT-PCR testing is not available and patients have symptoms for more than 12 days, then the Clungene
® antibody test can diagnose most infected Covid-19 patients tested. If the test is negative a recommendation should be made to have a follow up RT-PCR test. New findings from a Michigan Medicine study confirm that antibody testing is predictive of prior COVID-19 infection, and rapid screening methods – even from finger pricks – are effective testing tools (
Michigan 2021).
In our study, 1 patient who had a negative RT-PCR test was enrolled and had IgM and IgG bands on our test. Upon reviewing the medical record, the patient was on Remdesivir and had bilateral infiltrates consistent with Covid-19 on chest imaging. Upon further investigation, the patient had a positive pre-hospital SARS-CoV-2 RT-PCR test. Combining PCR and antibody tests at Point-Of-Care can dramatically increase Covid-19 detection (
University of Cambridge 2020).
In addition, the ability of the Clungene
® antibody test to detect antibodies to the coronavirus’s spike protein’s receptor binding domain means it has the potential to assess the efficacy of most vaccines in development as well as convalescent plasma therapy. Recently international airports and American Blood Banks have been providing Covid-19 antibody testing services to determine whether a person has developed immunity to Covid-19 through vaccination or through contracting the virus previously (
McGlynn 2021;
The Blood Connection 2021). Limited evaluation of the Clungene antibody test has confirmed positive antibody test results following patients who have been vaccinated; additional study is needed to confirm the reliability of these results.
Limitations
Limitations of the study include a small sample size in 1 geographic area. The study also did not include special groups such as pregnant women or children. The subjectivity of symptom reporting by patients can be a confounding factor in determining the duration of illness. Some patients may have been symptomatic for a different time period than they recalled. To reduce patients’ burden and discomfort, we opted to use leftover blood from venipuncture in inpatient setting and finger pricks in outpatient setting to collect blood. We do not know if using 1 universal method of collecting blood would have made a difference in results.
The positive predictive value of any test is dependent on the prevalence of disease in the community. If a test for a disease has 90.5% sensitivity and 100% specificity, and the disease prevalence is 10%, the positive predictive value (PPV) is 100% and the negative predictive value (NPV) is 98.95% (in a population of 10 000 people, 905 tests will be positive, and 95 of those will be false). If the disease prevalence is 50%, the PPV will be 100% and the NPV will be 91.3% (in a population of 10 000 people, 4525 tests will be positive, and 47 550 of those will be false). In areas where there is little Covid-19 community spread, the test may be suboptimal and result in a false sense of security regarding the level of immunity in the population. We understand that our findings may not be replicated in settings where seroprevalence of disease may be less than 50% as we expect in hospitalized patients. The positivity rate for the nares SARS-CoV-2 RT-PCR tests ranged between 3.5% to 10% in the Sharpe healthcare system.