Introduction
Chronic granulomatous disease (CGD) is one of the most common primary immunodeficiencies of childhood, often diagnosed within the first 3 years of life (
Kuhns et al. 2010;
Battersby et al. 2013;
Thomsen et al. 2016). It is caused by defects in the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex, required for phagocytic production of reactive oxygen species (ROS) and subsequent intracellular killing of bacteria and fungi. Any mutation within the genes encoding the 5 subunits of the NADPH oxidase, namely
CYBB (gp91
phox),
NCF1 (p47
phox),
NCF2 (p67
phox),
NCF4 (p40
phox), and
CYBA (p22
phox), resulting in functional impairment of ROS production, can lead to CGD (
Matute et al. 2009;
Roos and de Boer 2014).
Patients commonly present with lower respiratory tract infections, acute or recurrent bacterial and fungal infections, or failure to thrive (
Guide et al. 2003;
Thomsen et al. 2016). Due to both neutrophil dysfunction and dysregulation of the immune system, affected individuals are predisposed to granuloma formation, hyperinflammation, and autoimmunity (
Cale et al. 2000;
Winkelstein et al. 2000;
De Ravin et al. 2008). Manifestations in the gastrointestinal tract (early onset inflammatory bowel disease) can be another indication of CGD (
Marciano et al. 2004). Although symptoms often appear during infancy, in some, presentation occurs during late childhood or adulthood and is associated with residual function of the NADPH oxidase system (
Köker et al. 2013). Patients with CGD may also be affected by other auto-inflammatory disorders, including systemic lupus erythematous, arthritis, or vasculitis. Osteomyelitis is occasionally seen as well. Dysfunctional autophagy and inflammasome activation as well as inadequate resolution of inflammation are thought to contribute to the pathophysiology of CGD.
Defects in NADPH oxidase confer infectious susceptibility to a surprisingly limited spectrum of microbes; specifically, those that are catalase-positive (
Kaplan et al. 1968;
Lazarus and Neu 1975). Catalase degrades host-derived hydrogen peroxide, thereby limiting the availability of ROS to kill phagocytosed organisms. For this reason, infections of the lung associated with
Aspergillus fumigatus account for up to 40% of cases, while infections stemming from
Staphylococcus aureus and
Burkholderia cepacia are also common. Liver abscesses occur in almost 35% of cases, with skin and lymph node infection taking place in approximately 20% of patients. In North America, the majority of serious infections are caused by
Staphylococcus aureus,
Serratia marcescens,
Burkholderia cepacia complex,
Aspergillus species,
Nocardia species, and
Klebsiella species, although involvement of other microorganisms have also been reported.
Importantly, inflammatory manifestations in the absence of underlying infection can affect a variety of organs and tissues, including the brain, lungs, liver, spleen, gastrointestinal tract, genitourinary tract, skin, and lymph nodes (
Marciano et al. 2004;
Mahdaviani et al. 2013). Non-caseating granulomatous lesions composed of multinucleated giant cells are a classic finding in patients with CGD (
Schappi et al. 2008). In the lungs, liver, and spleen, such lesions may flare and resolve spontaneously, without intervention. Thus, examination by imaging (radiography, ultrasound, computed tomography, magnetic resonance), alongside biopsy and tissue or fluid cultures are essential to identify the extent and severity of infections as well as the microorganism responsible. These modalities also help to guide the management of inflammatory complications.
Once clinical suspicion of CGD arises, the diagnosis is investigated by assessing the functional activity of NADPH oxidase. This can be achieved by direct measurement of superoxide (for example, nitroblue tetrazolium (NBT) reduction) or by flow cytometric analysis of dihydrorhodamine (DHR) oxidation, which measures neutrophil oxidative burst function. The latter method provides a ratio (termed neutrophil oxidative burst index, NOBI) of oxidative function in neutrophils of the suspected case versus healthy control.
Genetic diagnosis of CGD is considered standard of care and is essential for prognosis, long term care planning, and family counselling. Mutations in
CYBB, located on the X chromosome and thus conferring X-linked recessive inheritance, account for up to 65% of CGD cases in North America. In contrast, an estimated 25% of cases stem from biallelic mutations in
NCF1, which is inherited in an autosomal recessive manner. Mutations in
CYBA,
NCF2, and
NCF4 represent <5% of CGD cases. X-linked CGD is considered more clinically severe, with earlier presentation and more severe infections than autosomal recessive CGD (
Winkelstein et al. 2000;
Kuhns et al. 2010).
Female carriers of a single
CYBB mutation have impaired NADPH oxidase activity in only a portion of phagocytes as a result of lyonization. This active carrier state can lead to a range of symptoms, including aphthous ulcers, arthralgia, and cutaneous photosensitivity (
Battersby et al. 2013). Infectious complications are known to be highly likely in patients with low residual neutrophil oxidative burst function (
Kuhns et al. 2010;
Marciano et al. 2018).
Prophylactic treatment with trimethoprim-sulfamethoxazole and an azole antifungal agent, such as itraconazole, is the most effective modality of treatment to reduce the frequency of potentially fatal bacterial and fungal infections. It is important to note that despite appropriate prophylaxis, many patients still experience at least 1 severe bacterial or fungal infection every 3–4 years (
Roos and de Boer 2014).
Currently, allogeneic hematopoietic stem cell transplantation (HSCT) is the only curative option for patients with CGD (
Ho et al. 1996;
Seger et al. 2002;
Ahlin et al. 2013). The HSCT event-free survival rate for CGD patients is estimated >80%, with outcomes likely to continue to improve over time (
Connelly et al. 2018). In contrast, results of gene therapy trials have so far been discouraging due to associated myelodysplastic syndrome, difficulty achieving long-term engraftment, and complications such as oligoclonal expansion (
Kohn 2010;
Kaufmann et al. 2014).
Here, we present radiographic findings of 10 pediatric patients who were diagnosed with CGD. These patients were followed in our centre over a period of 10 years. Seven had an X-linked form of CGD with confirmed mutations in
CYBB, the gene encoding gp91
phox. Three patients had autosomal recessive CGD; 2 of them with mutations in
NCF1 encoding subunit p47
phox of NADPH oxidase, and 1 with a rare mutation in
NCF2, which encodes p67
phox. Patient characteristics are summarized in
Table 1.
Discussion
As illustrated in this case series, invasive bacterial and fungal infections as well as inflammatory complications are a significant threat for CGD patients. Radiological assessment of patients who present with recurrent infection is extremely helpful for guiding diagnosis and monitoring the response for treatment (
Towbin and Chaves 2010). Very often, these cases require biopsy or culture to isolate the causative microorganism. Together, this can further guide antimicrobial and antifungal therapy, and help clinicians to better understand the underlying immunodeficiency.
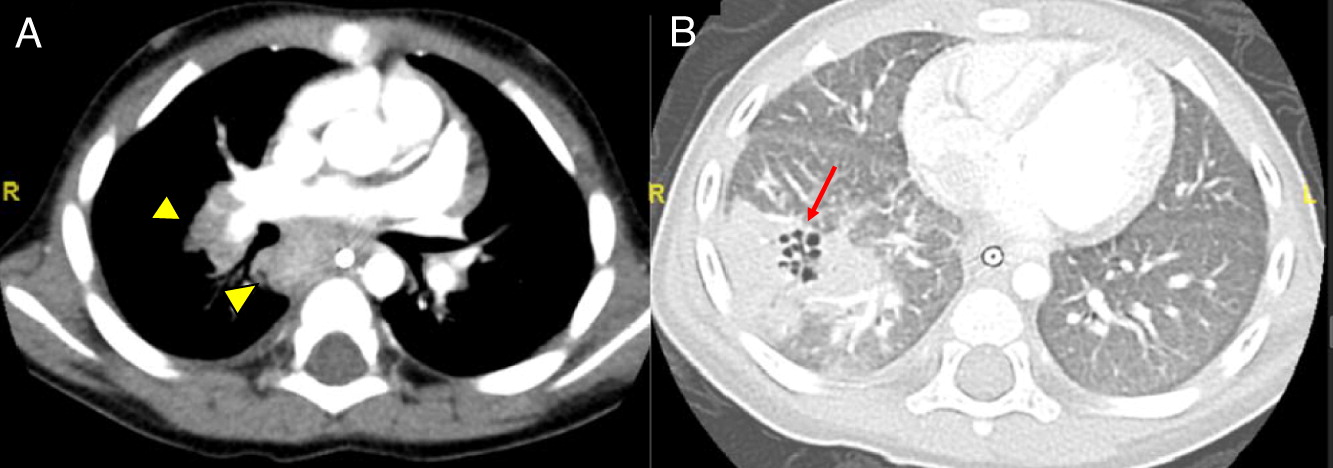
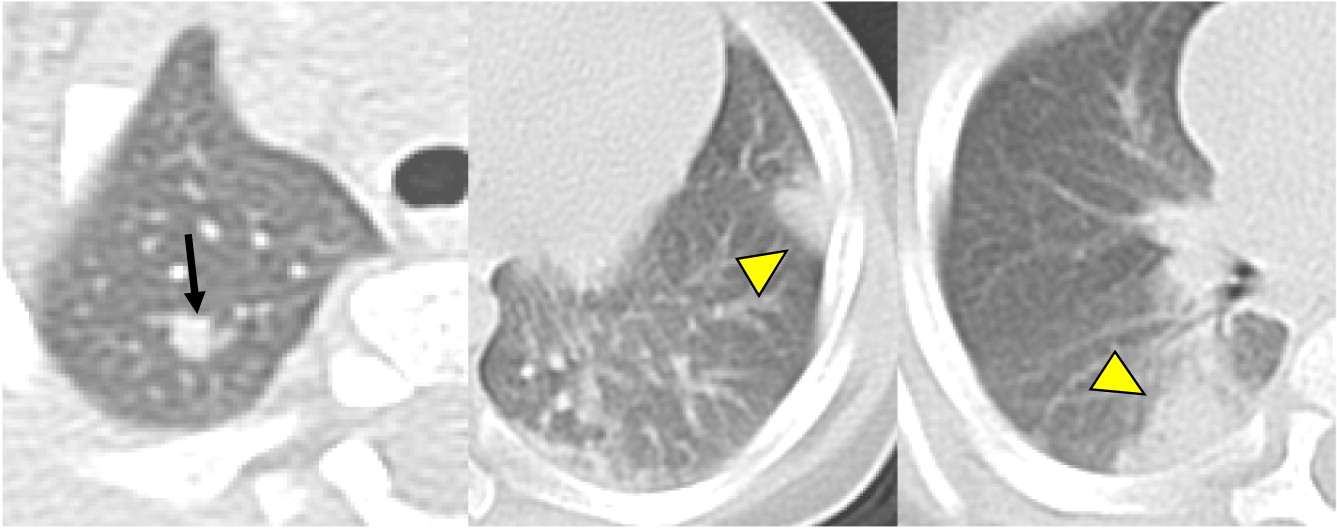
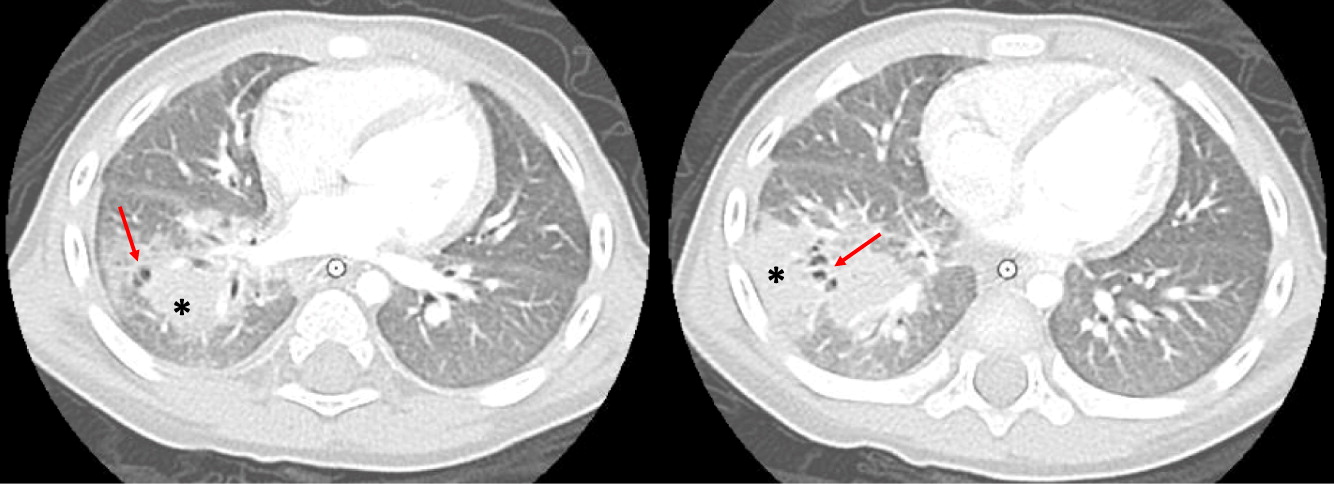
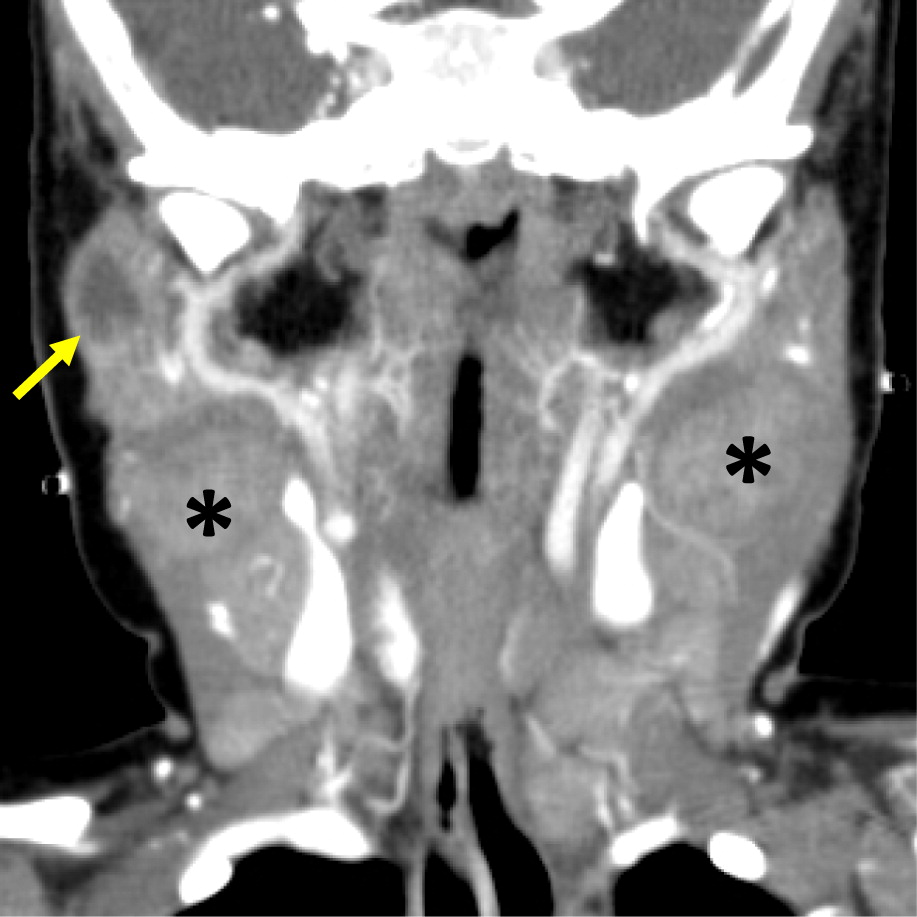
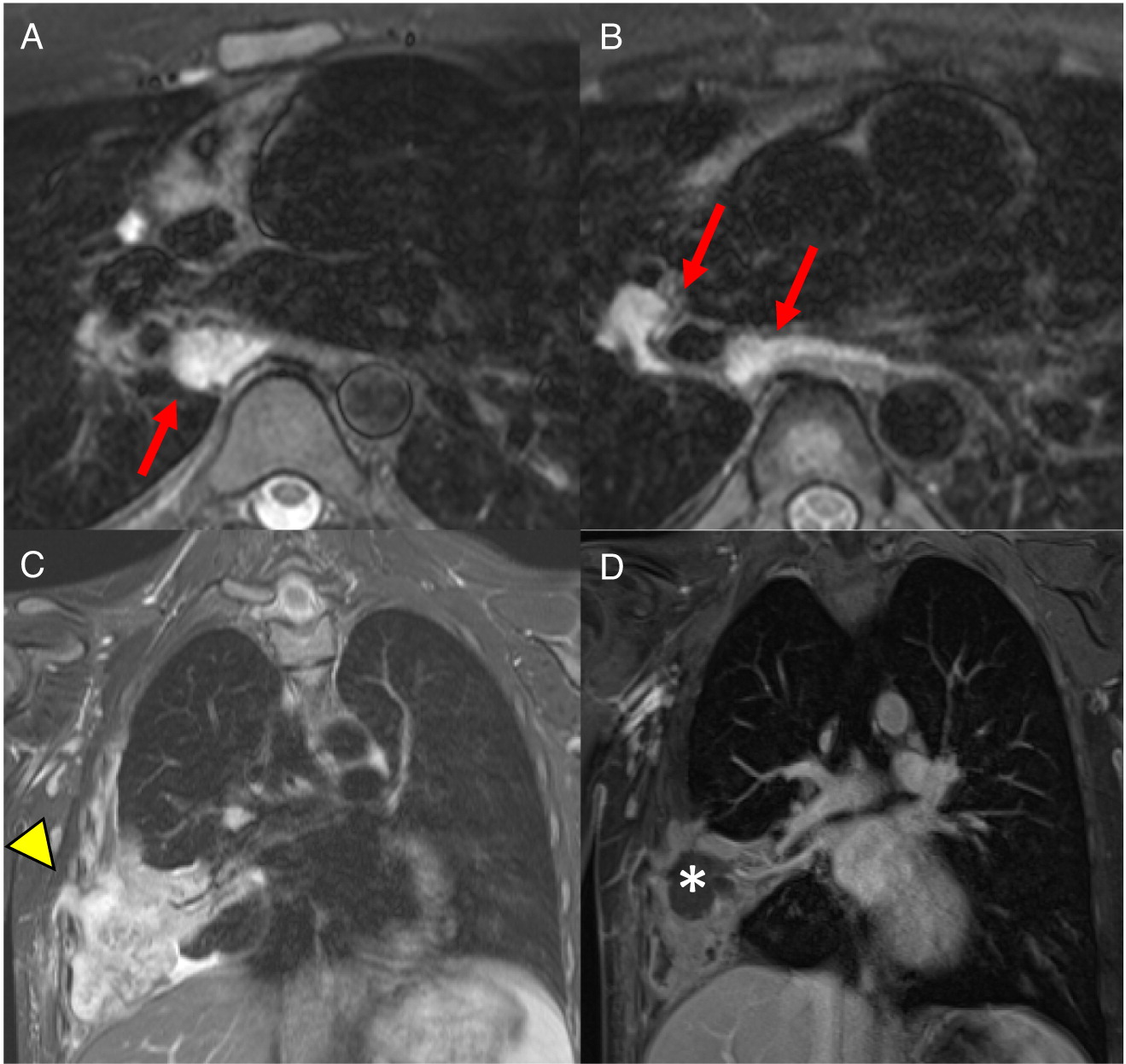
Imaging of the lungs is one of the most important modalities to consider. In this case series, 8 of 10 patients had pulmonary manifestations such as pulmonary nodule, abscess, cavitation or hilar lymphadenopathy. A combination of these findings in any 1 patient is suggestive of underlying immunodeficiency. As many as 80% of CGD patients present with pulmonary complications and this remains a primary cause of death in non-transplanted patients (
Winkelstein et al. 2000). Chest radiographs may show a variety of findings including consolidations, reticulonodular opacities, bronchiectasis, and scarring. CT scans may also identify consolidation, ground-glass opacities, centrilobular nodules, bronchiectasis, septal thickening, air trapping, or scarring. Complications such as abscess formation, cavitary lesions, empyema, or lung fibrosis may also be shown. There have also been reports of spreading to the chest wall and associated osteomyelitis of ribs and vertebrae (
Towbin and Chaves 2010;
Mahdaviani et al. 2013).
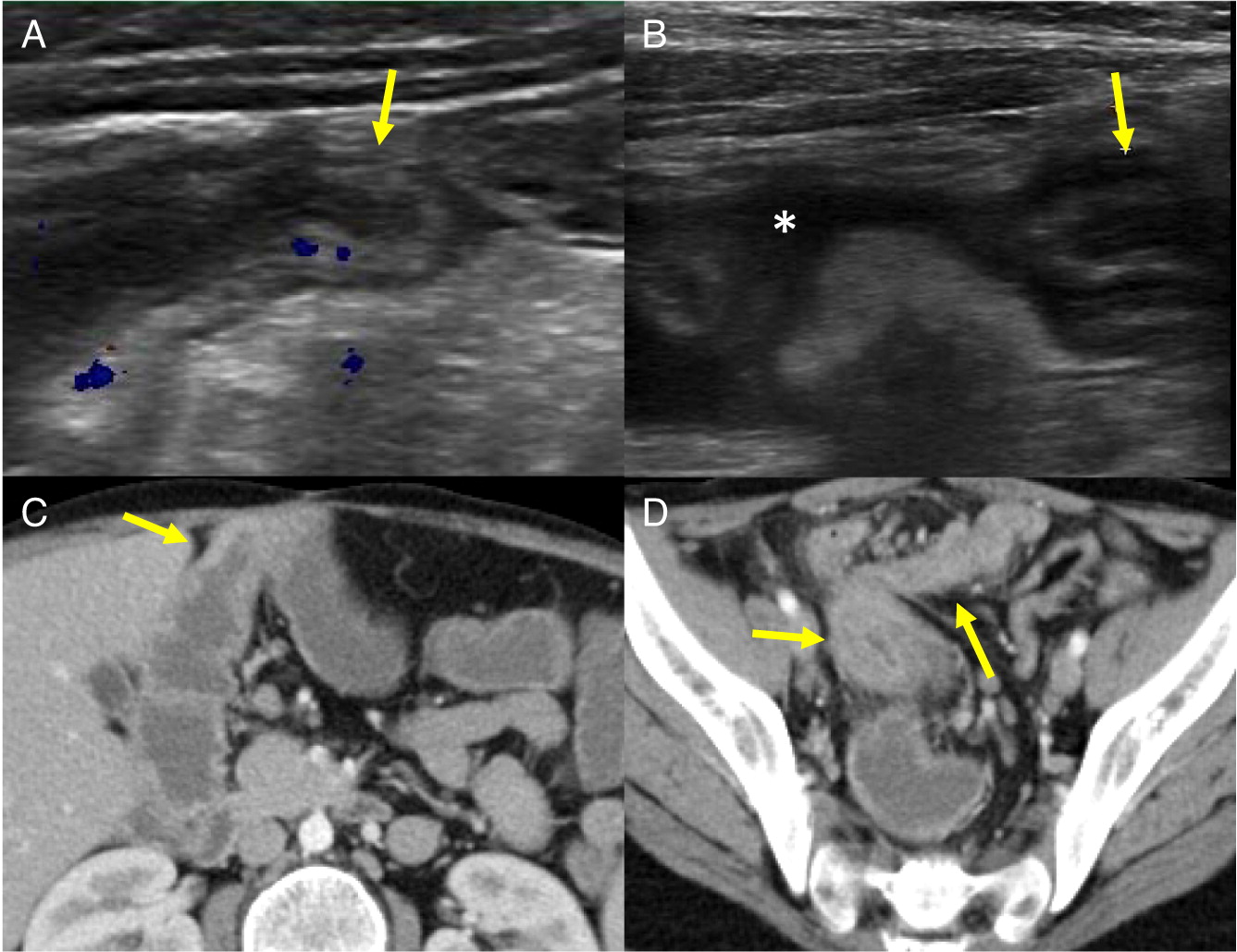
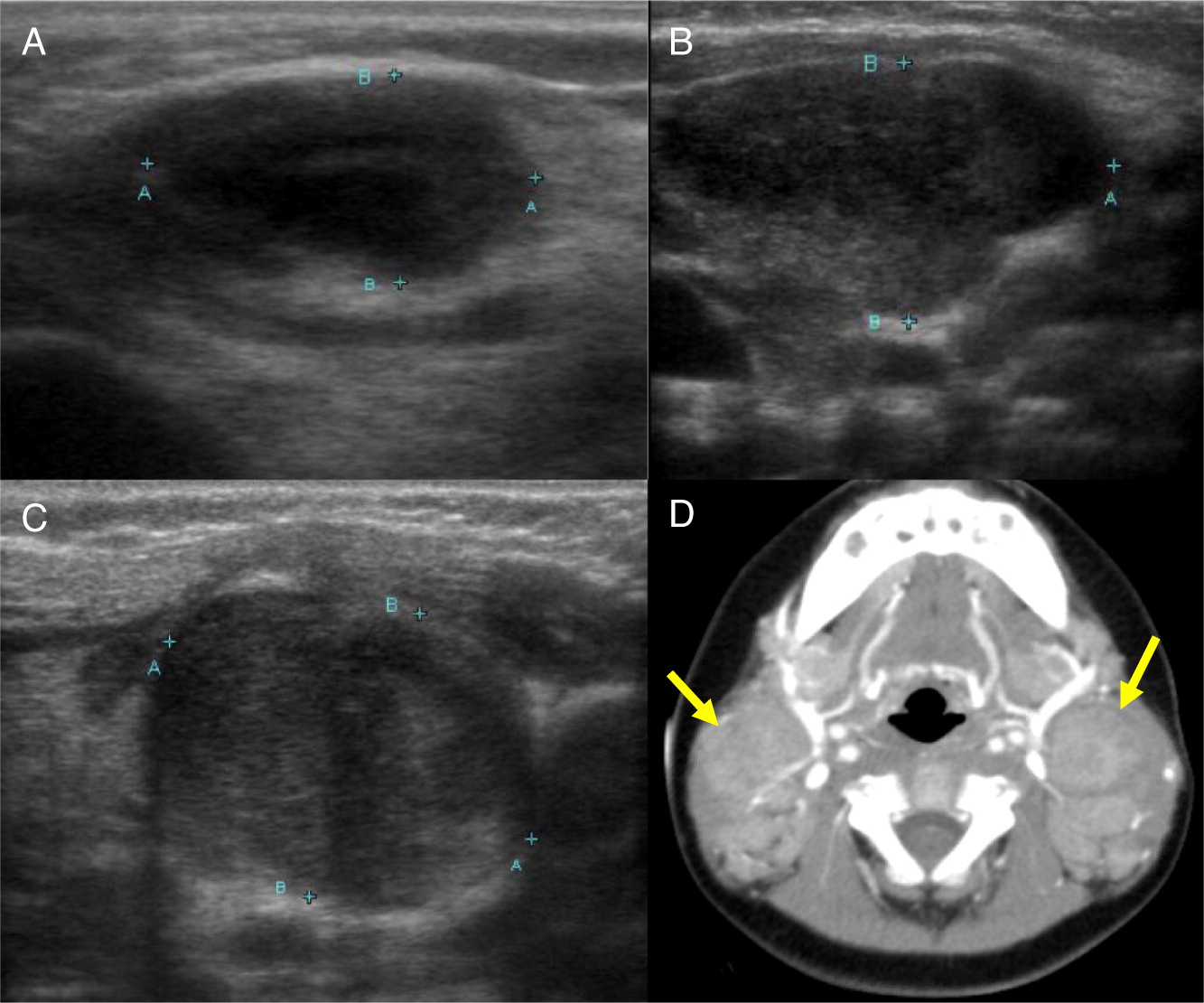
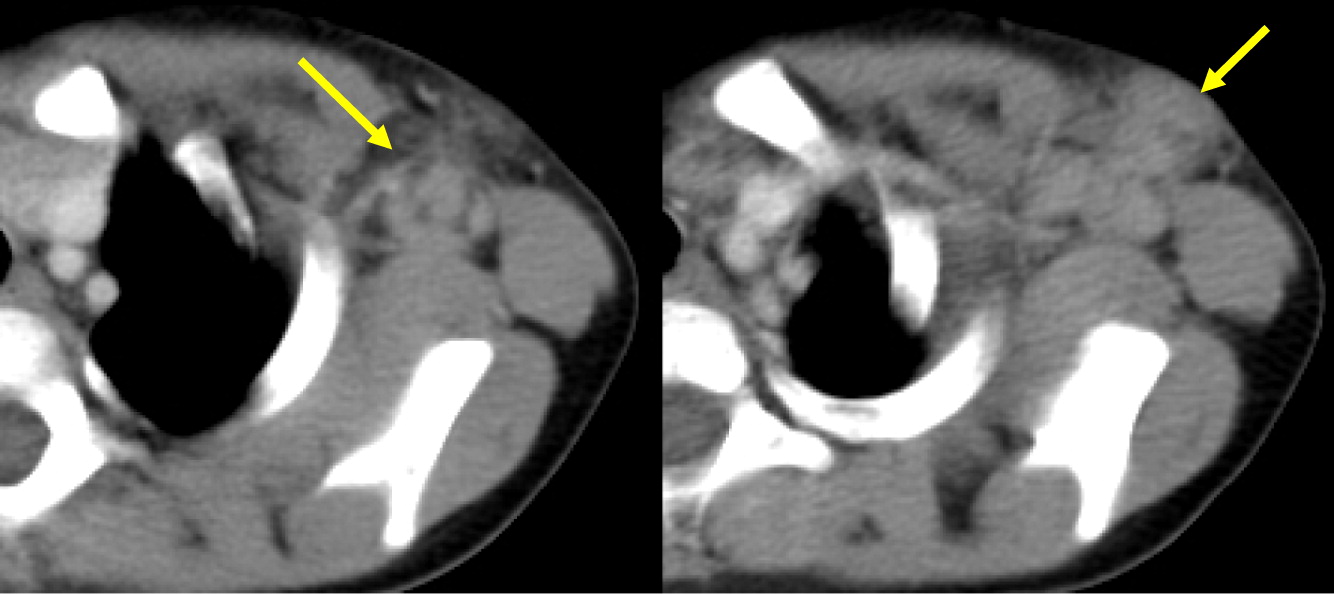
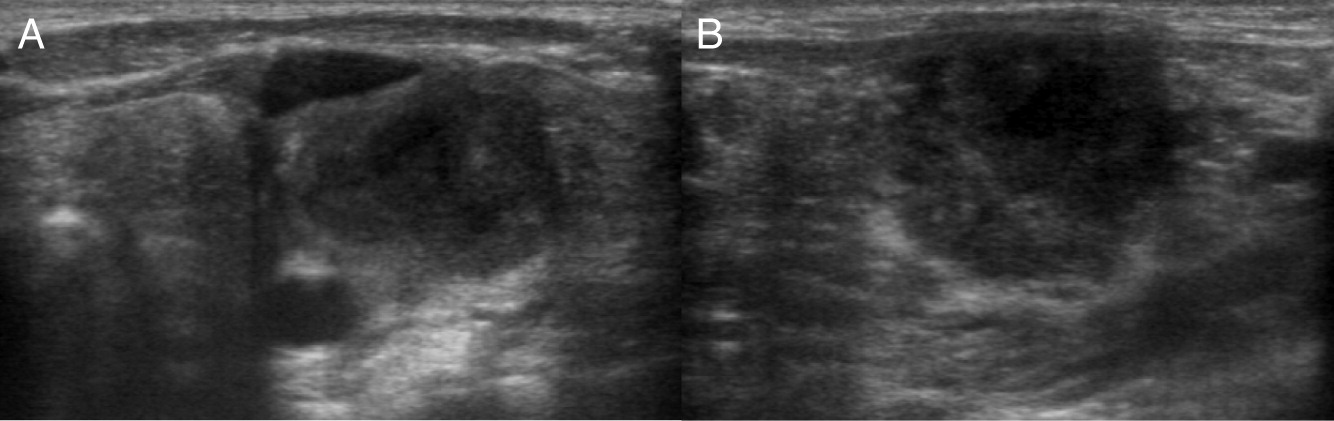
Changes in lymph nodes, particularly lymphadenopathy, lymphadenitis, and granuloma formation was the second most common finding in the CGD patients reviewed here. The cervical chain is most frequently involved, alongside hilar and mediastinal lymph nodes.
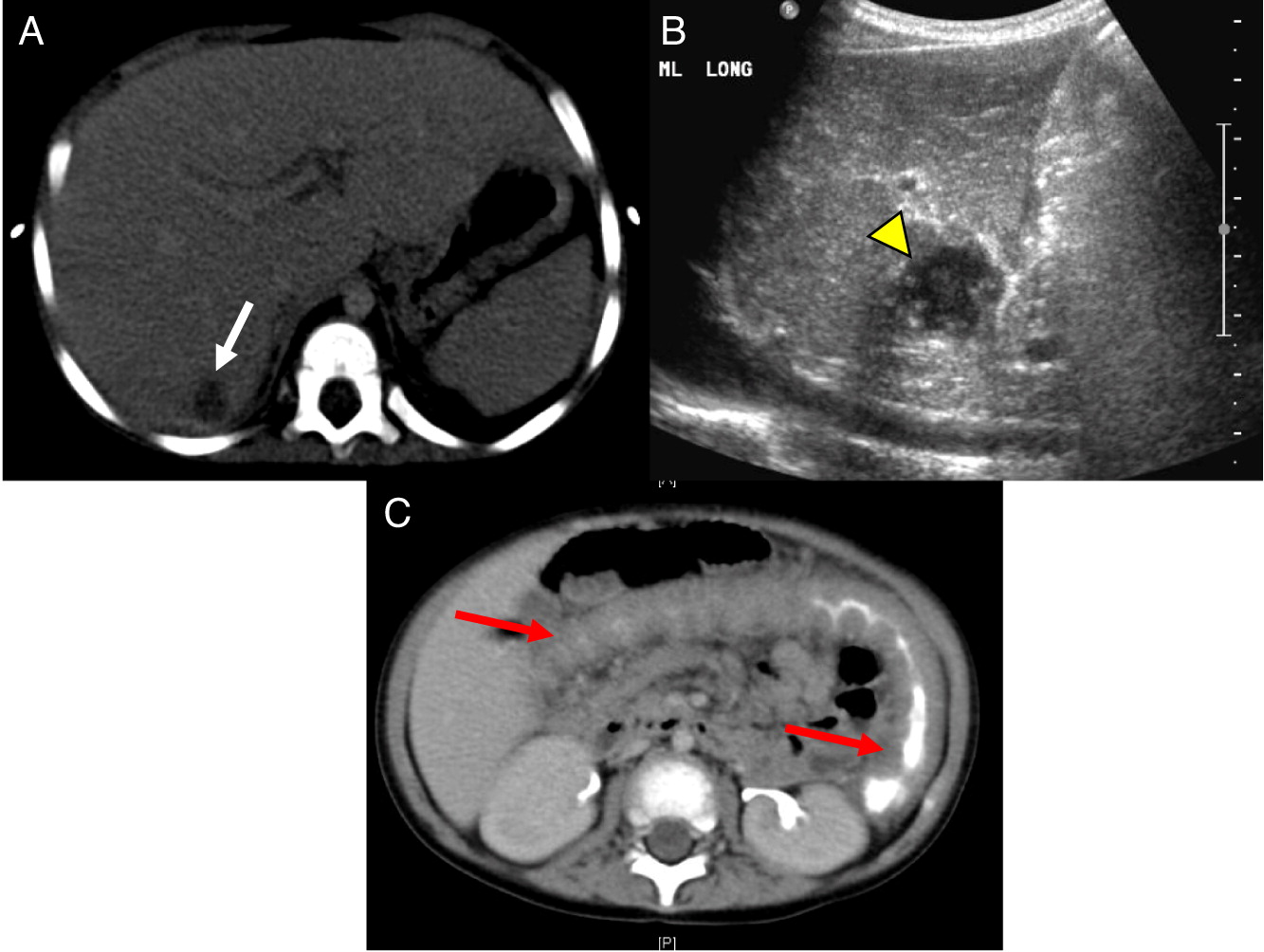
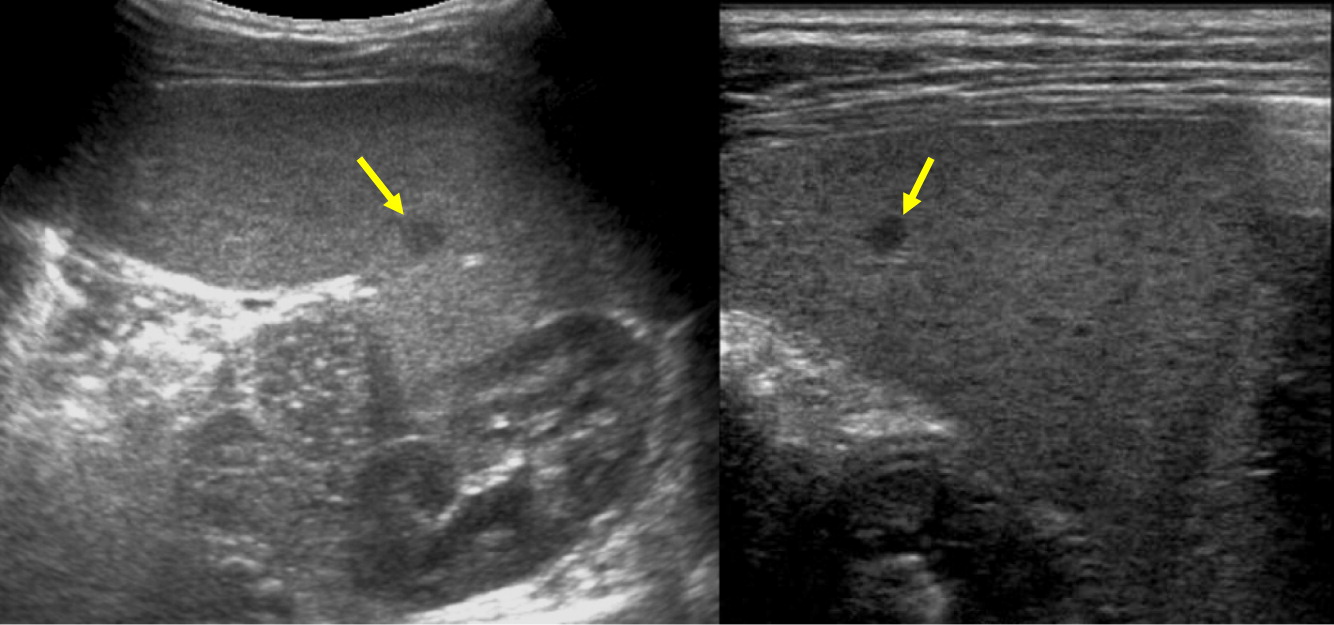
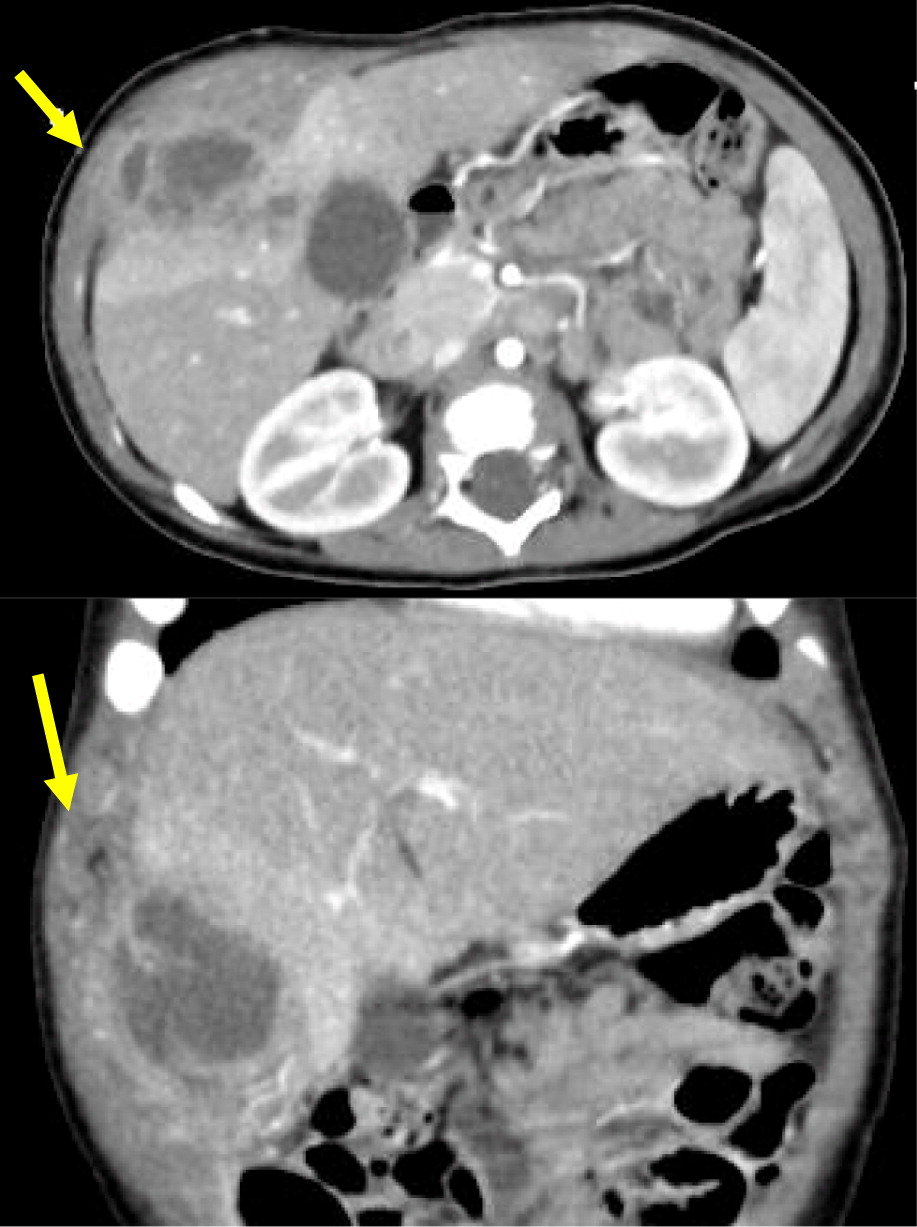
Hepatic abscesses or granulomas are often found in the liver, and are present in 25%–50% patients. Although liver lesions are more common in the adult population, they can found in pediatric patients as well. In our patients, 2 had liver abscesses and (or) granuloma. As demonstrated in our cohort, such lesions may also affect the spleen, and tend to wax and wane over time, generally disappearing or shrinking after HSCT (
Winkelstein et al. 2000;
Towbin and Chaves 2010).
The most common imaging findings of the GI tract are bowel wall thickening and inflammation mimicking IBD. In our patients, 3 out of 10 had GI manifestations identified upon biopsy or bowel imaging. These vary from granuloma formation to non-specific bowel wall thickening or inflammation. The age of presentation (early onset IBD) and presence of pigmented macrophages are 2 important clues for diagnosis of CGD. Interestingly, the muscle lesions seen in one of our patients is not frequently reported (
Murguia-Favela and Manson 2014).
To date, advancements in antimicrobial and antifungal prophylaxis have been essential for improving the morbidity and mortality of patients with CGD. Radiographic and pathological findings continue to be essential for monitoring disease progression, while genetic testing of the patient and family members can aid in the determination of carrier status. Furthermore, tests that evaluate the level of residual NADPH function and thus severity of disease can be used to identify patients who would benefit from HSCT.