Introduction
Chronic mucocutaneous candidiasis (CMCC) is characterized by chronic, non-invasive
Candida spp. infections of the skin, nails, and mucus membranes (
Ahonen et al. 1990;
Puel et al. 2011). Interleukin (IL)-17-producing T cells have been demonstrated to play a critical role in fighting off bacterial and fungal infections (
Puel et al. 2012). The cytokine IL-17 can be further characterized into 6 different types, IL-17 A-F, which can bind to 5 unique receptors, termed IL-17R A-E. The IL-17RA is the common subunit for all 5 members of the IL-17 receptor family (
Okada et al. 2016).
Genetic aberrations which can disrupt the differentiation of lymphocytes into Th17 effector cells, or production and binding of IL-17, can result in a CMCC phenotype. These include upstream players responsible for the production of IL-17 producing cells, such as the adaptor protein caspase recruitment domain-containing protein 9 (CARD9) or transcription factors like signal transducer and activator of transcription 1 and 3 (STAT1 and STAT3) (
Glocker et al. 2009;
Minegishi et al. 2009;
Liu et al. 2011). Furthermore, mutations in the autoimmune regulator (
AIRE) gene have previously been associated with neutralizing autoantibodies against IL-17A, IL-17F and (or) IL-22, as well as syndromic CMCC due to impaired IL-17 mediated immunity (
Puel et al. 2010). Downstream of IL-17RA, patients with mutations in the intracellular signaling SEF/EL-17R (SEFIR) domain of the adaptor molecule ACT1 (
Zhang et al. 2014), which is crucial for IL-17 signaling, also suffer from CMCC (
Boisson et al. 2013). Collectively, these findings suggest that CMCC is caused by mutations which impair the IL-17 pathway.
Given that neutralizing antibodies against IL-17 cytokines can result in CMCC phenotypes (
Kisand et al. 2010;
Sarkadi et al. 2014), it is thus plausible that genetic aberrations affecting IL-17 cytokines and receptors could also cause this syndrome (
Puel et al. 2010). IL-17 is secreted from TH17 cells in the presence of microbial antigens, resulting in the recruitment of IL-17RA expressing neutrophils to the site of infection (
Nahum 2017). This process is thought to contribute to cutaneous immunity against
C. albicans and
S. aureus infections. The clinical and immunological features of a cohort of 21 patients with autosomal recessive mutations in
IL17RA was recently described (
Levy et al. 2016). Affected individuals were most commonly of Middle Eastern, Japanese or South American ancestry. Interestingly, they did not present with classic CMCC—only a minority of patients had nail candidiasis. However, all patients suffered oral thrush and scalp or skin
Candida spp. infections, as well as
S. aureus skin infections. The staphylococcal infections presented in the form of folliculitis, furunculosis and pustulosis. There have also been reports of sinopulmonary infections as well as pulmonary tuberculosis (
Levy et al. 2016;
Okada et al. 2016).
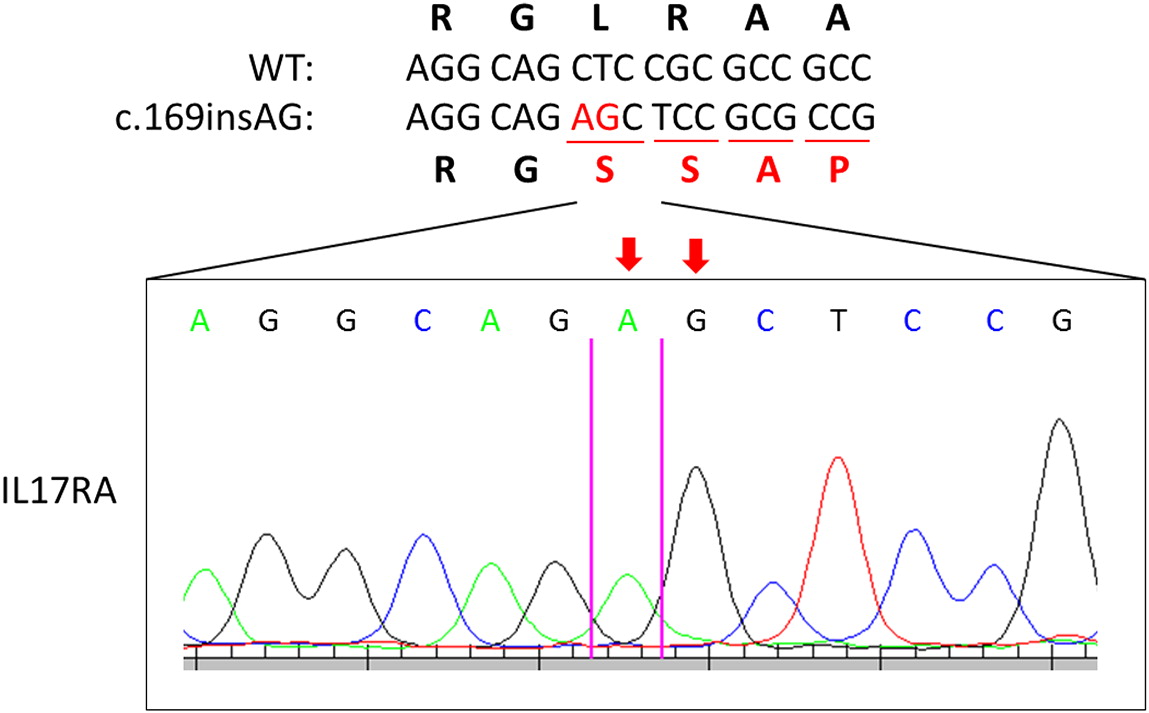
We report here a patient who presented with recurrent oral thrush, a history of eczema complicated with recurrent superinfection of S. aureus and 2 episodes of sinopulmonary infections. When utilizing whole exome sequencing, we identified a novel homozygous mutation in IL17RA.
Methods
Ethics
Patient informed consent was obtained, and information collected prospectively and retrospectively from medical records (REB Protocol no. 100005598, The Hospital for Sick Children).
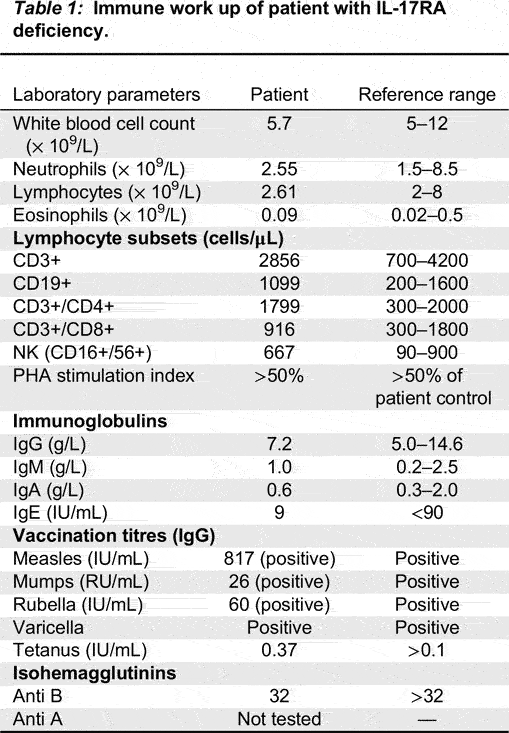
Serum concentration of immunoglobulin and specific antibodies
Levels serum concentrations of immunoglobulins (IgG, IgA and IgM) were measured by nephelometry and IgE concentrations determined by RIA using the IgE PRIST kit (Pharmacia Diagnostics, Dorval, QC, Canada). Serum antibodies to tetanus toxoid were measured by ELISA (Binding Site, Birmingham, UK). Serum antibodies to measles, mumps and rubella were measured using ELISA kits available from Euroimmun (Gross Groenau, Germany). Isohemagglutinin titres were determined by 2-fold serial dilution with erythrocytes, and are reported as antiglobulin phase, the dilution at which agglutination occurs.
T and B cell proliferative response
Lymphocyte proliferative responses to the mitogen phytohemagglutinin (PHA) was performed in triplicate and compared with simultaneously stimulated normal controls, as previously described (
Arpaia et al. 1994).
Exome sequencing and variant calling
Exome library preparation and sequencing was performed by The Centre for Applied Genomics (TCAG), Toronto, ON, Canada. DNA was quantified by Qubit DNA HS assay (Life Technologies, Carlsbad, CA, USA) and 100 ng of input DNA used for library preparation using the Ion AmpliSeq Exome Kit (Life Technologies). The Ampliseq Exome library was immobilized on Ion PI™ Ion Sphere™ particles using the Ion PI Template OT2 200 Kit v3. Sequencing was performed with the Ion PI Sequencing 200 Kit v3 and Ion PI Chip v2 in the Ion Proton™ semiconductor sequencing system in accordance with the manufacturer’s instructions. Alignment and variant calling were performed using Torrent Suite (v4.0) on the Ion Proton Server, using the Ion Proton ampliseq germline low stringency setting and the hg19 reference genome. The variants were annotated using an in-house annotation pipeline (
Stavropoulos et al. 2016).
Sanger sequencing analysis
Genomic DNA was extracted from isolated peripheral blood lymphocytes using the Geneaid Genomic DNA Mini Kit. Genomic DNA was amplified by PCR with specific primers designed upstream and downstream of the IL17RA gene. Sequencing was performed using GenomeLab Dye Terminator Cycle Sequencing (DTCS) Quick Start Kit (Beckman Coulter) and analyzed on CEQ 8000 Genetic Analysis System (Beckman Coulter).
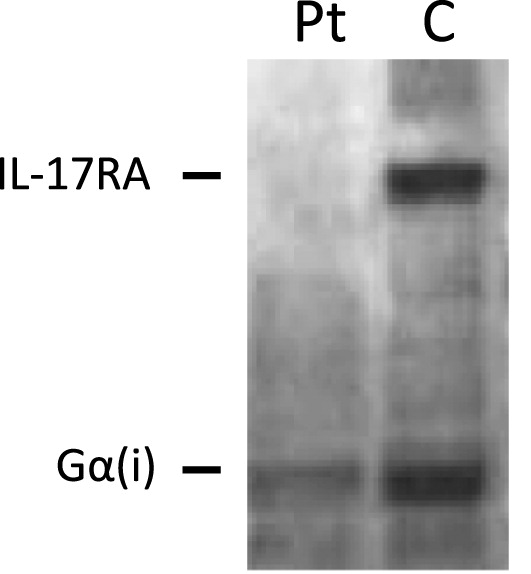
Western blotting
Whole blood was collected by venipuncture and peripheral blood mononuclear cells isolated by Ficoll separation. Whole cell lysates were prepared in 1% TritonX-100 buffer and protein expression of IL-17RA analyzed by Western blotting. Primary anti-IL17RA and goat anti-rat secondary antibodies were purchased from Abcam.
Discussion
We report here a child presenting with recurrent S. aureus skin infections, atopy, and limited oral thrush and diaper rash. He was initially thought to have an autosomal dominant mutation given his father’s history of autoimmunity; however, genetic analysis of STAT1 and AIRE were negative. Further assessment by whole exome genome sequencing revealed a novel homozygous mutation in IL17RA, encoding a crucial receptor involved in IL-17 signaling.
Patients affected with
IL17RA mutations are categorized under the umbrella of CMCC, however, they do not present with classic CMCC—only a minority have skin and nail candidiasis (
Levy et al. 2016;
Okada et al. 2016). Our patient’s recurrent
S. aureus infections were initially thought to be secondary to a leaky skin barrier due to his atopic dermatitis. Thus, his diagnosis was delayed until years later when next generation sequencing technologies became more readily accessible.
Clinically, genetic aberrations in
IL17RA are associated with susceptibility to oral thrush and scalp or skin
Candida spp. infections, as well as
S. aureus skin infections. The staphylococcal infections present in the form of folliculitis, furunculosis, and pustulosis. There have also been reports of sinopulmonary infections (
Levy et al. 2016;
Okada et al. 2016). Together, our patient’s clinical picture fits with the reported phenotypes in the literature.
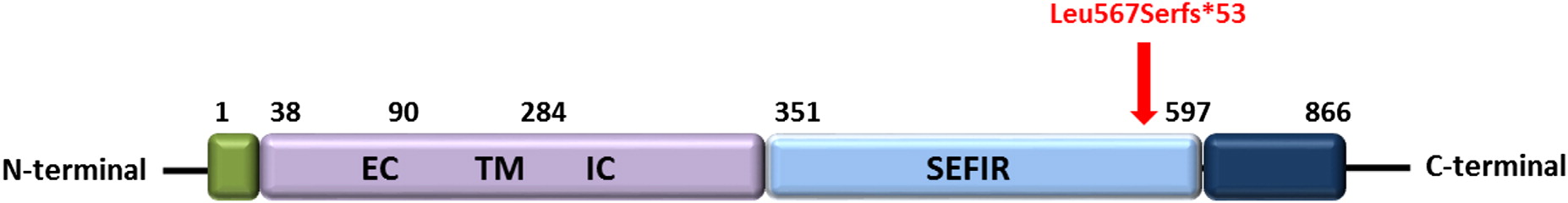
IL17RA encodes IL-17RA, a type 1 membrane glycoprotein that forms one half of a heterodimer required for IL-17A and IL-17F signaling (
Gaffen 2009). The mutation identified in our patient is localized to the SEFIR domain, which is essential for interaction with ACT1 and allows for IL-17RA dependent downstream effects (
Maitra et al. 2007;
Ho et al. 2010). Structure-function studies have demonstrated that the SEFIR motif is critical for IL-17A dependant activation of pathways, including MAPK, NF-κB, and C/EBP (
Maitra et al. 2007).
Levy et al. (2016) previously reported on a kindred with a frameshift mutation (p.N440Rfs*50) affecting the same SEFIR domain as our patient. The reported patients’ clinical picture resembled our patient as they also suffered from eczema and had skin pustulosis and folliculitis. The null mutation was associated with abolished lymphocyte responses to IL-17, as well as susceptibility to recurrent staphylococcal skin infections and bacterial respiratory infections, consistent with our patient.
IL-17A plays an important role in the clearance of
S. aureus (
Ishigame et al. 2009;
Cho et al. 2010). Using murine models of IL-17A deficiency,
Ishigame et al. (2009) showed increased susceptibility of
Il17a−/− mice to opportunistic
S. aureus infection compared to wild-type mice. However, intravenous administration of the bacterium revealed no difference in levels of bacterial dissemination, implying a role for IL-17A (and thus IL-17RA) in controlling local but not systemic
S. aureus infections. In our patient, the localized infection at the skin and mucosal membranes may be partially accounted for by the dependence of keratinocytes and bronchial epithelial cells on this cytokine’s protective effects. Further, IL-17A (alongside IL-17F) regulates immune function through stimulation of, among others, antimicrobial peptide production (
Ouyang et al. 2008). The absence of functional IL-17RA results in abolished responses to the cytokine IL-17A (
Minegishi et al. 2009). Mechanistically, this supports the chronic
S. aureus skin infections that our patient suffered from.
Clinically, this case presented a diagnostic challenge. While the patient suffered from chronic oral thrush and a diaper rash, he also had eczema, skin staphylococcal infections and other oral lesions. Because both S. aureus and Candida spp. are typically commensal organisms found in healthy individuals, these infections can occur after insults to the skin, such as eczema. Therefore, it is possible that the infections in our patient were actually secondary to the initial skin lesions. Moreover, the lack of autoimmune manifestations as well as the limited fungal infection did not support the diagnosis of typical CMCC associated with AIRE and STAT1 mutations. However, this patient did ultimately have concerning features for immunodeficiency which warranted completing whole genome sequencing in an attempt to identify a genetic culprit. Specifically, in our patient with the phenotype of more than 1 recurrent and persistent infection of both Candida spp. and S. aureus, requiring multiple treatments of both oral and topical anti-microbial medications, and ultimately anti-fungal prophylaxis for 8 years, further genetic investigations were necessary when common culprit mutations for CMCC (AIRE and STAT1) were not identified. Together, this report highlights the need for comprehensive genetic analysis in all cases that present with recurrent fungal infections, regardless of whether the cause is thought to be primary or secondary.
In summary, we have described here a patient with recurrent CMCC and S. aureus infections due to a novel frameshift mutation in IL17RA affecting the SEFIR domain. Functionally, the absence of IL-17RA receptor expression confirms that this is a loss of function mutation.