A clinical trial protocol to evaluate the safety and pharmacokinetics of subcutaneously administered immunoglobulin in patients with primary immunodeficiency
Abstract
Introduction
Primary immunodeficiency
Immunoglobulin replacement therapy
Study rationale
Study objective
Primary pharmacokinetic objective
Secondary pharmacokinetic objective
Safety objective
Exploratory objectives
Investigational plan
Study duration
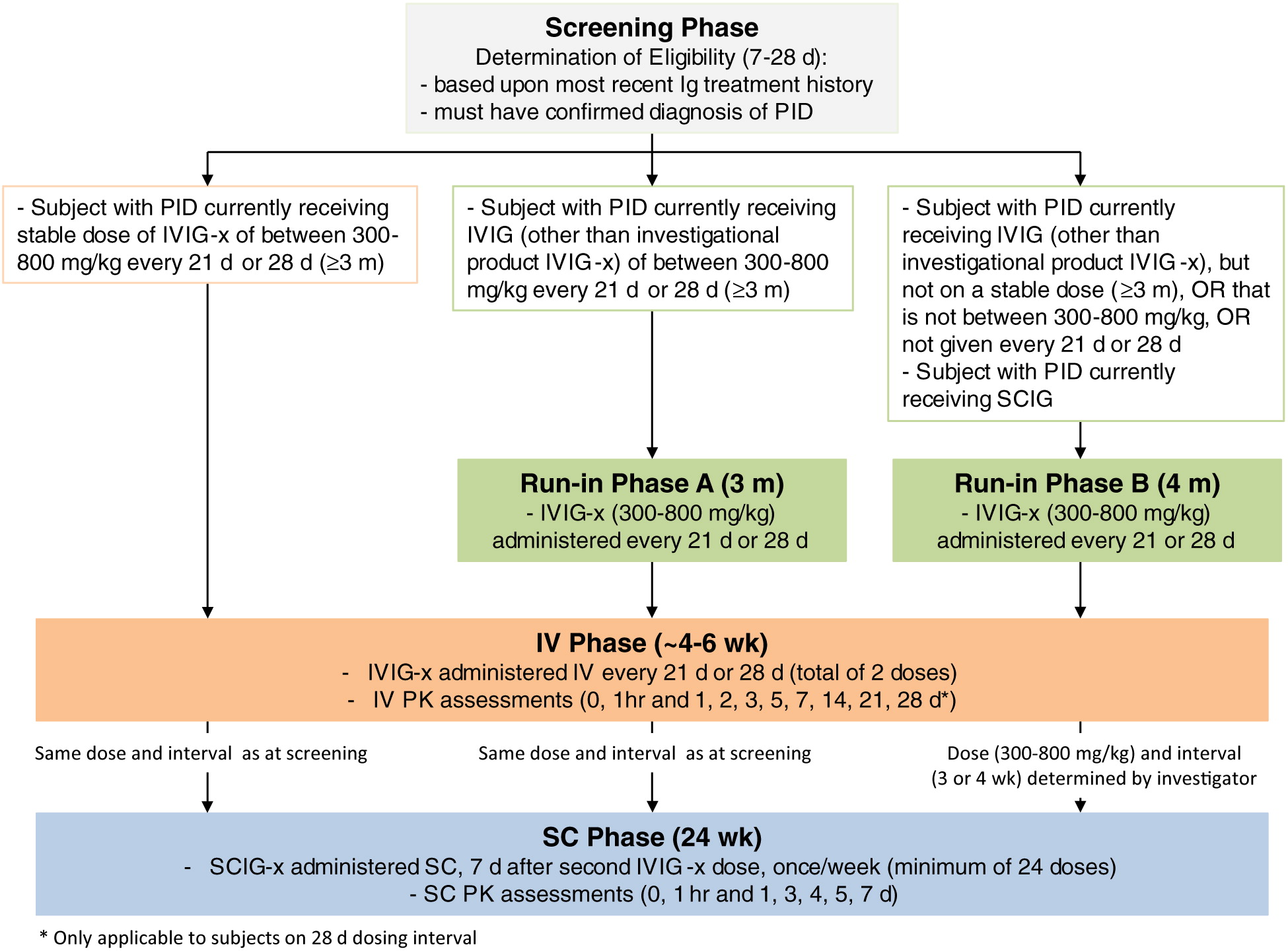
Study design
Run-in phase
IV phase
SC phase
Interim PK analysis
Investigators and study centres
Randomization and stratification
Blinding
Selection of the study population
Inclusion criteria
Exclusion criteria
Withdrawal of subjects
Premature termination of study/closure of centre
Study conduct
Treatments regimens
Treatment assignment
Subject identification
Physical examination
Medical history and demography
Subject diary
Dosage
Subcutaneous administration
Visit schedule
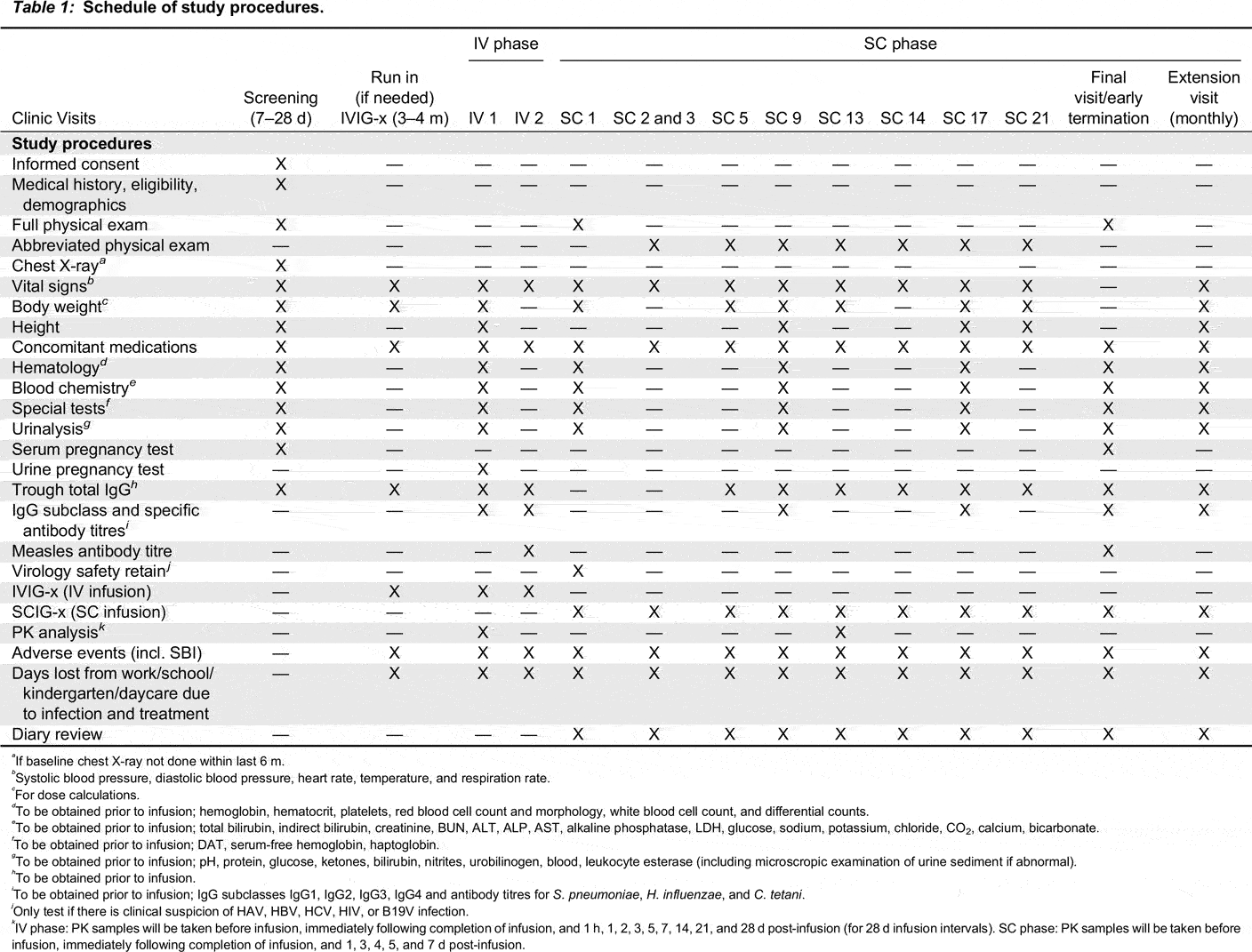
Screening: day 28 or day 21 to day 7
Run-in phase (if required)
IV phase, baseline visit 1 and PK assessment
IV phase, visit 2
SC phase, infusion week 1
SC phase, infusion weeks 2 and 3
SC phase, infusion weeks 4, 6, 7, 8, 10, 11, 12, 15, 16, 18, 19, 20, 22, 23, 24 (home setting)
SC phase, infusion weeks 5, 9, 14, 17, 21
SC phase, infusion week 13 and PK assessment
Completion of 24 weeks of SC infusions before PK analysis is complete
Final study visit/early termination visit
Assessment of pharmacokinetics
Primary PK objective
Secondary PK objective
Exploratory PK objectives
Assessment of efficacy
Assessment of safety
Safety parameters
REFERENCES
Information & Authors
Information
Published In

History
Copyright
Authors
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
There are no citations for this item
View Options
View options
Login options
Check if you access through your login credentials or your institution to get full access on this article.


