The leukocyte adhesion cascade system allows for leukocyte accumulation at sites of tissue inflammation and infection. Adhesion molecules including selectins, integrins, and members of the immunoglobulin superfamily of proteins, are expressed on leukocytes and vascular endothelial cells and are required for leukocytes to migrate from the vasculature into the tissues (
Schmidt et al. 2013). Leukocyte adhesion deficiency (LAD) syndromes are primary immunodeficiency disorders caused by defects in adhesion molecules on leukocytes resulting in impaired migration into tissues (
Al-Herz et al. 2011). LAD is divided into 3 subgroups including LAD-I (beta-2 integrin defect), LAD-II (fucosylated carbohydrate ligands for selectins are absent) and LAD-III (activation of all beta integrins is defective) (
Al-Herz et al. 2011). LAD-I is an autosomal recessive syndrome caused by a mutation in the integrin beta-2 gene (
ITGB2) resulting in deficiency and/or defects in CD18, the common beta chain of the beta-2 integrin family, and the inability of leukocytes to adhere to the endothelium and migrate into tissues (
van de Vijver et al. 2012). LAD-I has also been associated with impaired inhibition of interleukin-23 and interleukin-17 resulting in a hyperinflammatory response and chronic inflammation (
Moutsopoulos et al. 2014). Patients with severe LAD-I, defined as less than 2% of CD18 expression, typically present in early infancy with recurrent, life threatening infections that are frequently fatal before age 2 years old without hematopoietic stem cell transplant (HSCT) (
Almarza Novoa et al. 2018). Patients with mild to moderate LAD-I, defined as 2%–30% of CD18 expression, tend to have fewer significant infections and often survive into adulthood without HSCT (
Almarza Novoa et al. 2018). Common clinical manifestations of LAD-I include delayed separation of the umbilical cord with omphalitis, recurrent bacterial infections especially of skin and mucus membranes, impaired wound healing, absent pus formation, periodontitis, and leukocytosis (
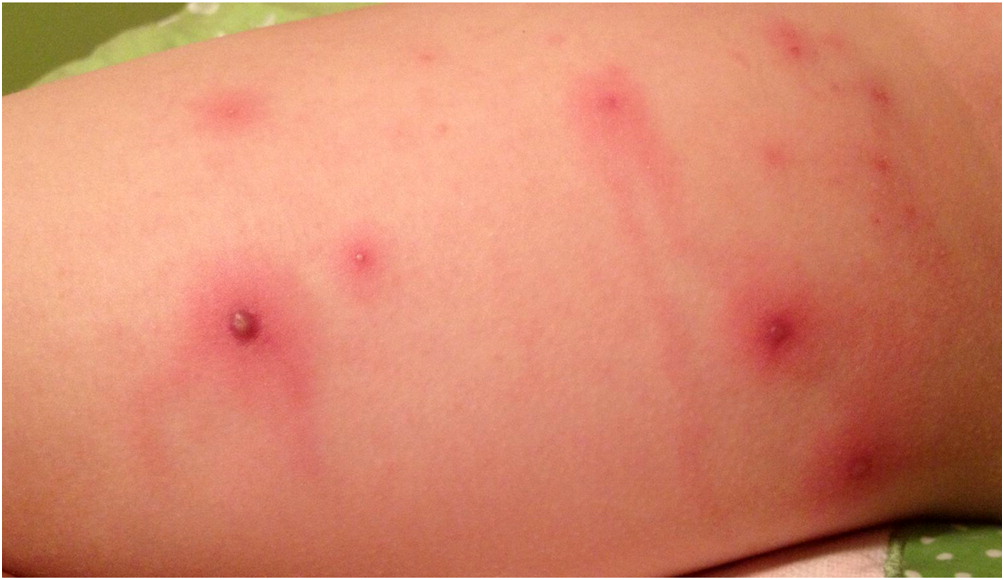
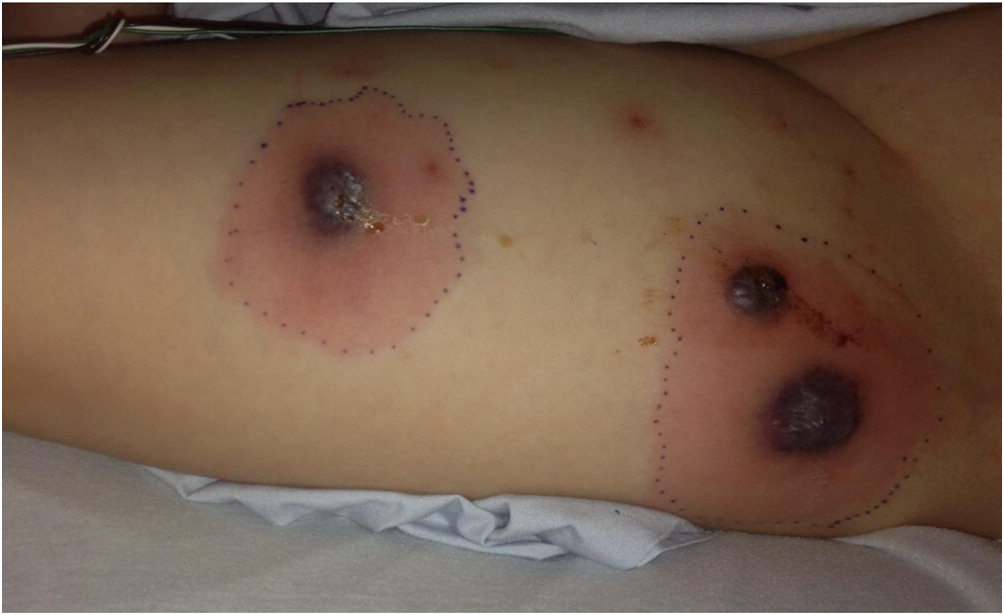
Hanna and Etzioni 2012). We describe 2 siblings with LAD-I caused by a novel mutation in
ITGB2 and atypical presentation including pyoderma gangrenosum (PG).