Pathophysiology
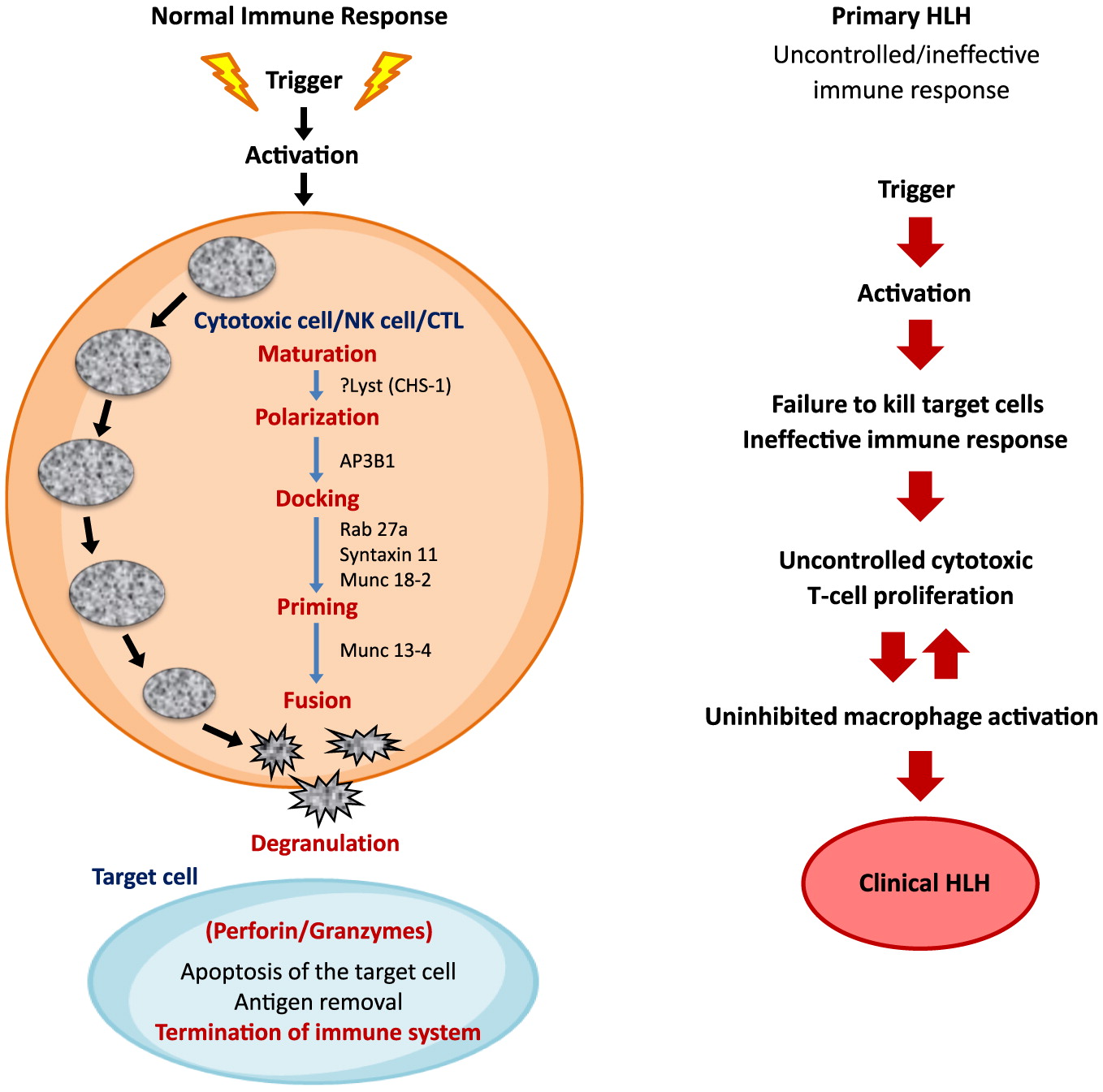
The histological hallmark of HLH is an accumulation of histiocytes in various tissues along with defective cytotoxic function. In immunocompetent individuals, cytotoxic T-lymphocytes (CTL), natural killer (NK) cells, and T-regulatory cells are critical for the control of infectious and inflammatory responses. The innate immune system acts as the first line of defense against infections. It is mediated by phagocytes, neutrophils, and NK cells which together initiate phagocytosis, antigen presentation, and activation of the adaptive immune system. The pathogen infected “target cells” are recognized by NK cells and CTLs. The CTLs, with helper T cell receptors (TCRs), initiate the cascade of immune response. This requires effective cell lysis through granule-mediated cytotoxicity. The granules are transported to the cell surface by the process of exocytosis, and are released into the target cells. Entry of the granules from activated CTLs and NK cells into the target cell is facilitated by perforin, which is produced by NK and CD8
+ T cells. Once perforin is liberated, pores are formed in the target cell membrane, allowing granzyme-B to pass into the target cell and induce cell apoptosis/death (
Figure 1). HLH arises from the impaired continuous stimulation of CD8
+ T cells, which in turn produces excessive INF-γ and further promotes uncontrolled secretion of other cytokines such as IL-1, IL-6, IL-10, IL-18, and TNF-α (
Janka and Lehmberg 2014). The defective function of NK cells and CTLs is permanent in primary HLH and transient in secondary HLH (
Henter et al. 1991c;
Faitelson and Grunebaum 2014;
Brisse et al. 2015).
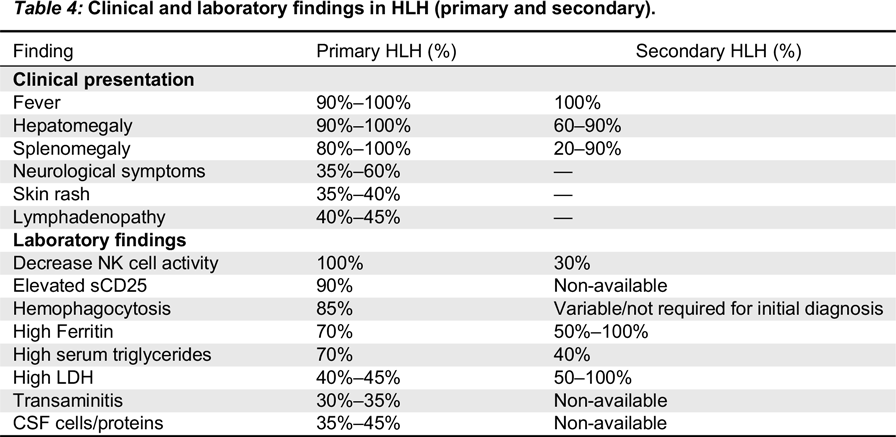
The circulating high concentrations of inflammatory cytokines and infiltration of the organs by activated lymphocytes and histiocytes manifest clinically as prolonged fever, hepatosplenomegaly, cytopenias, and (or) multi-organ failure (
George 2014;
Madkaikar et al. 2016). Fever is induced by IL-1, IL-6, and TNF-α. Similarly, pancytopenia is a result of suppressive activity of TNF-α and INF-γ, along with hemophagocytosis and elevated transaminases, bilirubin, and lactate dehydrogenase (LDH). Low concentration of fibrinogen in blood is a result of high circulating levels of plasminogen activator secreted by the activated macrophages. This further stimulates plasmin, consequently causing hyperfibrinolysis. High concentration of triglyceride is a result of the suppressive actions of TNF-α on lipoprotein lipase. Similarly, activated histiocytes/macrophages lead to hyperfibrinolysis resulting in high serum ferritin (
Janka and Lehmberg 2014;
Yang et al. 2016). Growth differentiation factor (
GDF15)-mediated upregulation of ferroportins may also contribute in hyperferritinemia (
Wu et al. 2013).
Primary HLH
Clinically, primary HLH is indistinguishable from the secondary form. In some cases, the diagnosis of FHLH is presumed by presentation at an early age, unremitting course or multiple reactivations, and presence of a similar history in one or more family members (
Arico et al. 1996).
Genetic HLH
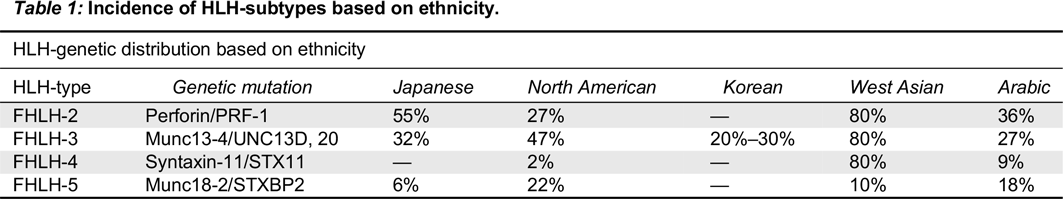
FHL-1, linked to chromosome 9q21.3-22, was first described in 4 Pakistani and a Saudi family. However, the corresponding gene and associated protein remained unidentified (
Ohadi et al. 1999). In 1999, Stepp et al. described the association of perforin gene mutation to HLH (FHLH-2) (
Stepp et al. 1999). Subsequently, 3 more genetic defects have been identified which play a key role in the cytotoxic granule pathway (FHL-3, 4, and 5) (
Feldmann et al. 2003;
zur Stadt et al. 2005;
Côte et al. 2009;
Macartney et al. 2011). All the above mutations are transmitted in an autosomal recessive manner. A report from Zhang et al. describes synergistic defects of different disease-causing mutations leading to FHLH, suggesting a potential digenic mode of inheritance (
Zhang et al. 2014).
Other inherited immune deficiency syndromes associated with life-threatening HLH include pigmentary and non-pigmentary disorders. The pigmentary immune deficiency disorders include Griscelli syndrome-type 2 (GS-2), Chediak-Higashi syndrome-1(CHS-1), and Hermansky-Pudlak syndrome-2 (HPS-2) are linked to
RAB27A,
LYST, and
AP3B1 genetic mutations, respectively. CHS-1 presents with oculocutaneous albinism, easy bruising, and associated frequent bacterial infections. The clinical picture of HLH in CHS-1 is described as “accelerated phase”, mimicking acquired HLH (
Janka 2007a). HPS-2 is characterized by oculocutaneous albinism, bleeding, and neutropenia (
Gholam et al. 2011).
The non-pigmentary immune deficiency syndromes associated with HLH are mainly X-linked lymphoproliferative (XLP) disorders. The genetic mutation of XLP-1 is linked to the Src-homology 2 domain-containing gene (
SH2D1A), a lymphocyte signaling adapter. XLP-2 is caused by a gene mutation in
BIRC4, which encodes X-linked inhibitor of apoptosis (XIAP). Both XLP-1 and XLP-2 syndromes have a high predilection to Epstein-Barr virus (EBV) infection, HLH, and dysgammaglobulinemia (
Yang et al. 2012). There is an association of lymphomas with XLP-1 but not XLP-2. Patients with XLP-2 develop colitis and can be at risk of being misdiagnosed with Crohn’s disease. A novel mutation in IL-2-ITK gene has been observed in few patients, both boys and girls with EBV-lymphoproliferative disease and HLH (
Ghosh et al. 2014). A recent review of 8 pediatric intensive care unit patients diagnosed with EBV-HLH found 50% to be associated with an ITK mutation, representing a very poor prognosis (
Zheng et al. 2016).
Primary immunodeficiency disorders (PID) and HLH
Although infrequent, HLH has been described in patients with different types of PID, particularly those related to genetic T cell, NK cell, and few B-cell/macrophage deficiencies. It is prudent to remember that acquired HLH patient might have underlying PID. In an international survey from centers involved in the diagnosis and treatment of primary immune deficiencies and/or HLH (Histiocyte Society, European Society of Blood and Bone Marrow Transplantation’s inborn errors Working Party, and the German Society for Pediatric Oncology and Hematology), 63 patients with PID were identified who fulfilled the diagnostic criteria for HLH. More than 80% of patients had 2 main groups of PID; combined immune deficiencies (CID) and chronic granulomatous disease (CGD). Some of the patients in the CID group developed HLH in the first months of life, at or before the diagnosis of immune deficiency. Most of the patients with CGD presented beyond the first year of life, some in early adulthood (
Bode et al. 2015).
In T cell defects, HLH is not associated with macrophage dysfunctions but primarily to T cell functional abnormality. HLH is seen in patients with classical severe combined immunodeficiency (SCID) lacking complete T cell function, which predisposes these patients to more infections and subsequent HLH. Regardless of the mechanism of immune dysfunction pertaining to B or T cells, clinicians should have a high index of suspicion to investigate for HLH or macrophage activation syndrome (MAS) (
Faitelson and Grunebaum 2014).
Treatment
Primary HLH without treatment is a rapidly fatal disease. In 1983, Janka reported 5% survival in a review of 121 cases (
Janka 1983). Early diagnosis and treatment has improved outcome considerably. Introduction of epipodophyllotoxin derivatives has especially improved the remission status of these patients. Etoposide was first reported in 1980 as a treatment for HLH among 3 children (
Ambruso et al. 1980). Plasma exchange was later attempted with transient resolution of symptoms in few patients (
Ladisch et al. 1982). A combination therapy including etoposide, steroids, intrathecal methotrexate and cranial radiation therapy in 1985 revealed prolonged remission in patients with FHLH (
Fischer et al. 1985). Henter et al. also reported prolonged remission with the combination therapy of teniposide in 7 children (
Henter et al. 1986). It is now well understood that FHLH is associated with defective apoptotic mechanisms (
Fadeel et al. 1999), and etoposide being very effective in initiating apoptosis, became the cornerstone of treatment in HLH (
Henter et al. 1997). In murine FHL, etoposide acts selectively on activated T cells and controls immune dysregulation (
Johnson et al. 2014). Similarly, the effect of corticosteroids can be explained by the anti-inflammatory and pro-apoptotic properties and cyclosporine A (CSA) in reducing T cell activity in HLH.
In 1994, the Histiocyte Society proposed a combination therapy comprising of etoposide, CSA and dexamethasone followed by HSCT for familial HLH. The regimen consisted of induction therapy with etoposide and dexamethasone, with gradual wean over 8 weeks followed by HSCT as soon as a suitable donor is available. In the absence of availability of a donor, continuation therapy was advised. Moreover, individuals with progressive CNS disease also received intrathecal methotrexate (
Henter et al. 1997). The outcome of children treated as per the Histiocyte Society’s HLH-1994 protocol was published in 2011, showing a 5-year survival rate of 54% ± 6%. This was even better in children who underwent HSCT (66% ± 8%) (
Trottestam et al. 2011). In their subsequent study (HLH-2004), the Histiocyte Society revised the HLH-94 protocol and added CSA upfront at the start of induction, instead of starting in the continuation phase, with an aim to get early remission. Recently, results of the HLH-2004 study have been published and have shown overall 62% (230/369) of patients were alive at the median follow up of 5.2 years. This study has also shown good results with the etoposide and dexamethasone combination therapy. However, there was no considerable improvement in overall outcome by adding upfront oral CSA (
Bergsten et al. 2017).
On the other hand, a second regimen adopted for over 15 years in Europe, comprised of an induction phase that included rabbit anti-thymocyte globulin (rATG) and methyl-prednisone (with gradual wean). This is followed by a continuation therapy consisting of cyclosporine and intermittent prednisone pulses until HSCT. Individuals with CNS involvement received a combination of intrathecal methotrexate and corticosteroids, similar to the HLH-94 protocol. The complete response was achieved in 73%, with partial response in 24% of patients (
Mahlaoui et al. 2007).
There are new therapeutic approaches to improve survival of patients with HLH. An innovative trial by a Cincinnati group,
Hybrid Immunotherapy in HLH, was recently completed in children <18 years of age. It comprised of rATG, etoposide, dexamethasone, intrathecal methotrexate, and hydrocortisone with a belief that a combination of two proven induction regimens (from the Histiocyte Society and Mahlaoui et al.) will result in comparable or even better remission rates. The evaluation of this trial is currently in progress (clinicaltrials.gov NCT01104025) (
Children’s Hospital Medical Center). Another clinical trial on tocilizumab (anti-IL-6 antibody) is underway. The excessive inflammation in HLH is cytokine driven, and standard modalities do not target cytokine storms directly in HLH. The aim of using Tocilizumab is to decrease cytokines in HLH (clinical trials.gov NCT02007239) (
Philadelphia). Anti-Interferon Gamma Monoclonal Antibody, N1-0501 (Novimmune), a phase 2 study is underway in the United States and Europe to evaluate safety and efficacy of N1-0501 in primary HLH. Unpublished data on 13 patients have shown satisfactory response in patients who failed first line therapy (
Jordan et al. 2015).
The first report of a successful HSCT in HLH was published in 1986 (
Fischer et al. 1986). A study of 86 children who were treated on the HLH-94 protocol followed by HSCT between 1995–2000 showed overall estimated survival rate of 64% (
Horne et al. 2005). In earlier reports, use of myeloablative conditioning regimens were associated with increased transplant related complications in the first 100 days post-transplant. This included veno-occlussive disease of the liver, respiratory complications, and overwhelming infections (
Horne et al. 2005;
Ouachee-Chardin et al. 2006). Subsequently, there has been increasing use of reduced intensity conditioning (RIC) regimens, which have improved the outcome in these patients. In 2010, Marsh et al. published their experience in 26 patients, and found 3-year probability of survival when treated with RIC (alemtuzumab, fludarabin and melphalan based regimen) at 92% (
Marsh et al. 2011). Comparable but slightly lower survival rates of 85% at 3-year median follow up in 25 patients treated with RIC was also reported by Cooper et al. (
Cooper et al. 2008).
Despite advances in management and treatment of HLH, approximately 25%–50% patients fail to achieve remission with conventional treatment, including HSCT, and require salvage therapy. Many agents have been used in HLH including rituximab, rATG, and alemtuzumab (
Mahlaoui et al. 2007;
Strout et al. 2010;
Chellapandian et al. 2013;
Marsh et al. 2013b). Rituximab, a monoclonal CD20-antibody has been used in patients with EBV-associated HLH with fairly well response. A retrospective study of 42 patients with EBV associated HLH, treated with rituximab in combination with conventional HLH-directed therapy, showed improved clinical and laboratory parameters (
Chellapandian et al. 2013). Similarly, when HLH becomes refractory to various immunosuppressive agents, it requires suppression of immune response at different levels, including cytotoxic CD8
+ T cells, NK cells, cytokine producing CD4
+ T cells, and antigen presenting cells. CD52 antigen is a small GPI-anchored protein expressed on lymphocytes, monocytes, macrophages, and dendritic cells. Alemtuzumab is a monoclonal antibody targeting CD52 antigen. Alemtuzumab has been reported as a bridging therapy to HSCT in 1 patient with refractory HLH (
Strout et al. 2010). A study by Marsh et al. involving 22 pediatric and adult patients treated with alemtuzumab experienced noticeable improvement in clinical symptoms and laboratory markers, and 77% survived to undergo allogeneic HSCT (
Marsh et al. 2013a).