Introduction
Familial haemophagocytic lymphohistiocytosis (FHL) is a rare autosomal recessive disorder with an incidence of 0.12 cases per 100 000 children per year. FHL is characterized by persistent hyperproliferation, hyperactivation, and infiltration of histiocytic and T-lymphocyte populations owing to systemic hypercytokinemia and defective lymphocytic cell cytotoxicity (
Henter et al. 1991;
Janka 2007;
Gholam et al. 2011;
Fisman 2013). The diagnosis of FHL is suggested by criteria including: prolonged fever, cytopenia, splenomegaly, hyper-triglicidemia or hypofibrinogenemia, hemophagocytosis, hyperferritinemia, and elevated soluble interleukin-2 receptor (sIL2R) blood levels (
Henter et al. 1998, 2004;
Janka and Schneider 2004;
Lee et al. 2004). Currently, 4 specific genetic defects that cause the majority of FHL cases are recognized and they are associated with CTL and NK cell cytotoxicity (
Rohr et al. 2010;
Gholam et al. 2011). Consequently, one of the most important pathogenic mechanisms in FHL patients is the impaired or absent natural killer (NK) and T-cell cytotoxicity, regardless of the normal quantity of cells (
Jordan et al. 2004;
Lee et al. 2004;
Janka 2007;
Rohr et al. 2010;
Gholam et al. 2011).
We present a report on a child who was suspected of having FHL due to a positive family history with severely reduced NK cell quantity, leading to an erroneous result of reduced NK cell function.
Materials and methods
The male patient was evaluated at 2 months of age for FHL because of his family history. Five siblings died within their first year of life owing to a severe hyper-inflammatory disease that occurred after an infection in the first weeks/months of life. In 1 of the deceased siblings a diagnosis of FHL was considered, with partial fulfillment of the diagnostic criteria for FHL; the evaluation was not completed as we decided to refer the patient for HSCT owing to clinically based suspicions. Genetic evaluation in this sibling showed absence of mutations in the perforin gene. No other genetic tests were available at that time. We therefore actively evaluated the patient for signs of FHL from birth. The parents are healthy first cousins of Arab Bedouin ethnicity. The patient was asymptomatic, and physical examination was unremarkable, without hepatosplenomegaly or rash. The complete blood count, cholesterol, and triglyceride levels were normal and the ferritin level was normal; however, the sIL2R was elevated at 3638 U/mL (normal 300–2000 U/mL). This abnormality taken together with the family history prompted the performance of an NK quantity and function assay. At 3 months of age, low NK (CD56+) number of 0.06 × 103 cells/µL (normal 0.3–0.7 × 103 cells/µL) was measured, constituting 1.9% of total lymphocytes (CD45+ gated). The rest of the lymphocytes subpopulation numbers were normal. Similar results were recorded at the age of 7 months, NK count was 0.11 × 103 cells/µL, constituting 1.4% of total lymphocytes.
Initial cytotoxicity functional assay of NK cells was performed at 3 months of age, using peripheral blood mononuclear cells (PBMCs) at the ratio of 50:1 Effector:Target K562 cells, which showed no activity (0% of specific lysis), whereas healthy control donors showed normal cytotoxicity (57.1% or 61.8% specific lysis). The amount of NK cells was still low, but slightly improved 4 months after the initial evaluation: 0.11 × 103 cell/ µL (normal 0.3–0.7 × 103 cells/µL).
The patient remained asymptomatic and was followed up with 2 positive criteria for FHL. Since we were hesitant to suggest a pre-emptive HSCT based solely on family history and these positive laboratory criteria for FHL (elevated sIL2R, NK cytopenia, and no NK cytotoxicity function), a further analysis of NK function was performed.
NK cells were isolated from the patient's peripheral blood, as well as from his parents and his healthy 4-year-old sister. In contrast to the routine lysis assay that uses PBMCs for the cytotoxicity assay, the target cells were mixed with a counted number of purified NK cells. The NK cells from the isolated fraction were counted by flow cytometry (CD56+, CD3−). In addition to the isolation, the cells were pre-activated overnight with 300 Units/mL of interleukin 2 (IL-2). NK cell isolation, cytotoxicity, and cytokine secretion assays were performed as previously described (
Hershkovitz et al. 2009;
Rosental et al. 2011).
This study was approved by the Soroka University Medical Center ethical committee.
Results
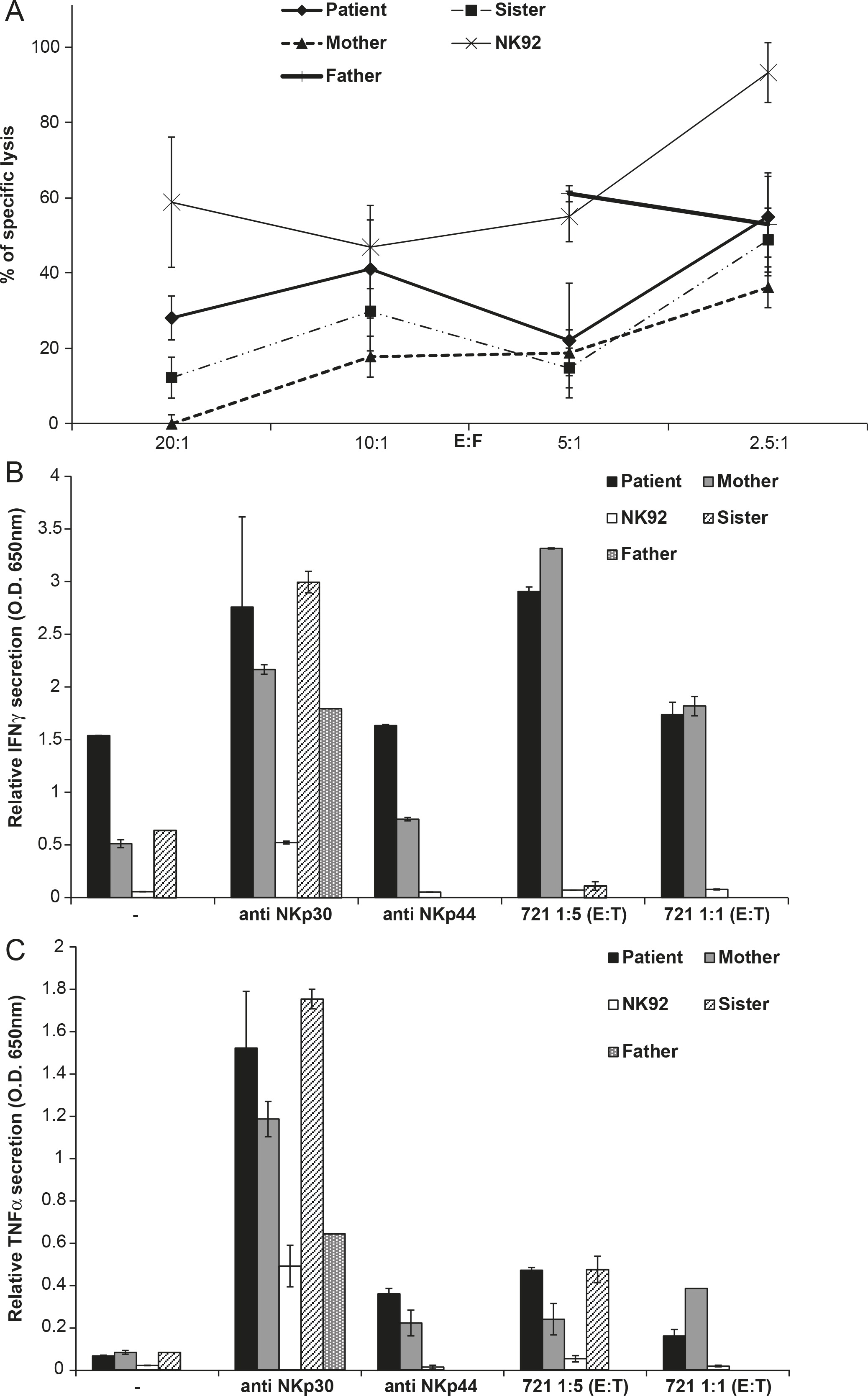
In the cytotoxicity assay, the patient's NK cells lysed the target cells (
Figure 1A). Additionally, the patient's NK-specific lysis was enhanced compared with his mother and it was slightly higher than his sister (
Figure 1A). The IFNγ and TNFα secretion by the patient's NK cells after a challenge with target 721 cells or anti-Natural Cytotoxicity Receptors (NKp30 and NKp44) antibodies showed levels that were close to his mother's and sister's NK secretion levels (Figures 1B and 1C). Owing to the low yield of NK cells from patient's father, the results for his NK cells are incomplete.
The patient did not undergo HSCT and continued to be followed. He is now 7 years old and thriving without signs of FHL. His last examination was in August 2012 for functionality of isolated NK cells. The results showed normal cytotoxicity, cytokine secretion, and CD107a up-regulation to the NK cell surface (data not shown).
Discussion
We present a case of an asymptomatic child with presumed FHL based on a positive family history and 2 diagnostic criteria (elevated sIL2R and decreased NK quantity and function). The initial NK function assessment did not take into account the extremely low levels of NK cells in peripheral blood, and it was considered to be without any NK cytotoxicity. Our extended workup showed that the purified NK cells possessed normal cytotoxic activity. Therefore the very low NK numbers hindered the NK cytotoxicity assay, but purification of the NK cells prior to the functional test allowed differentiation between the number of NK cells and their function. Decreased function and quantity of NK cells are one of the most important criteria for the diagnosis of FHL, and discounting the NK fraction when assessing NK function may cause an erroneous result, giving a false-negative NK function assay.
Cytotoxic function of human umbilical-cord-blood derived NK cells was shown to be lower than that of adult NK cells. Activation of these cord-blood NK cells and adult NK cells with IL-2 resulted in similar cytotxic activity of cord-blood NK cells to adult NK cells (
Le Garff-Tavernier et al. 2010). The first routine lysis assay did not include IL-2 activation, whereas the extended analysis included overnight IL-2 incubation. Therefore, this suggests that a complete NK cell assessment from very young infants or neonates should include activation with IL-2.
The diagnostic criteria for FHL are not specific; viral infections or malignancies in children without FHL can cause prolonged fever, cytopenia, hepatosplenomegaly, increased ferritin, and hemophagocytosis (
Imashuku 2000;
Janka 2007; Fisman 2013). Our patient did not have FHL; yet, with a positive family history and 2 criteria for FHL the diagnosis was not unlikely. Thus there is a large differential diagnosis of clinical presentations similar to FHL and this diagnosis should be done very carefully.
Because the previous 5 siblings became ill in the first months of life and succumbed in the first year, we can now safely assume on clinical grounds alone that our patient does not have the same disease and is not predisposed to developing FHL. The normal NK function also contributes to this assumption.
Our case report suggests that NK assessment should take into account the number of NK cells in peripheral blood. Either NK cell purification or correction for NK quantity should be considered when assessing NK function in peripheral blood, and purification of NK cells allows assessment of NK function even when very few NK cells are present. Activation of NK cells with IL-2 can enhance the credibility of NK cell function assessment in neonates and very young infants.
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work was supported by Israel Science Foundation grant 304/12 and by U.S./Israel Binational Science Foundation grant 2011123, granted to Dr. Angel Porgador. The work of Dr. Benyamin Rosental was supported by a postdoctoral fellowship from the Human Frontier Science Program Organization.