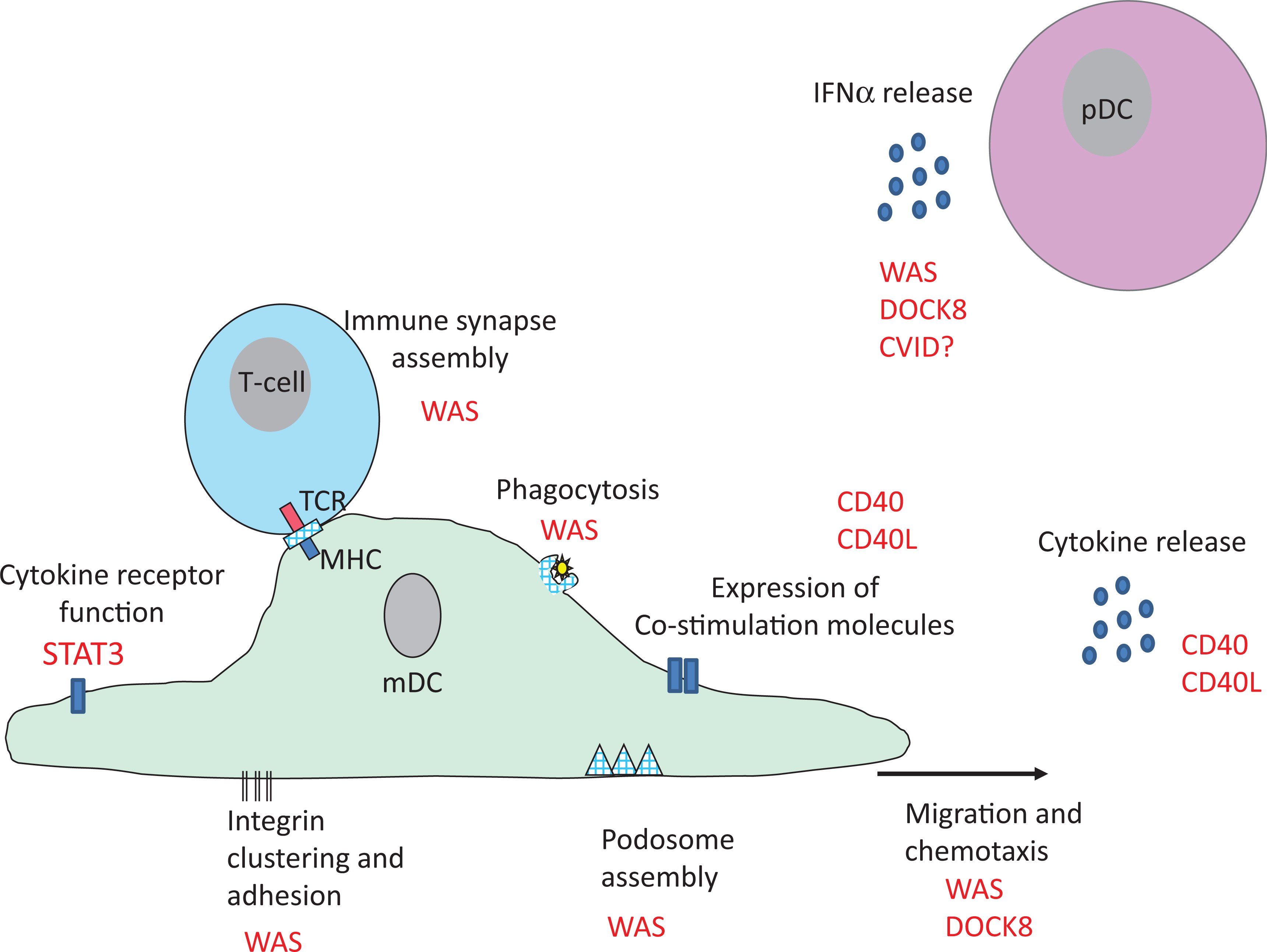
Probably the best studied PID with regard to DC is Wiskott–Aldrich syndrome (WAS): an X-linked condition that results from loss of function mutations in the
WAS gene (
Burns et al. 2004). WAS protein (WASp) is a cytoskeletal regulator that is expressed in all haematopoietic cells and is required for many cellular functions such as phagocytosis, cell migration, and immune synapse formation (
Thrasher and Burns 2010). Absence of WASp function results in a combined immunodeficiency that impairs the function of T, B, and NK cells as well as myeloid lineage cells. The clinical phenotype is broad as a result: characterized by recurrent bacterial, viral and fungal infections, autoimmunity, bleeding (as a result of platelet defects), severe eczema, and an increased risk of haematological malignancies.
Like other immune cells, DC rely on WASp for f-actin assembly (
Binks et al. 1998). It is therefore not that surprising that cytoskeletal-associated functions in DC are impaired by WASp deficiency. Initial studies focused on migration and demonstrated that mDC from patients (and mice) with WAS fail to form podosomes, F-actin rich structures that link the cytoskeleton to membrane integrins (
Binks et al. 1998;
Burns et al. 2001; Olivier et al. 2005). Cell adhesion and migration in vitro are impaired and the in vivo consequence is a failure of WASp-deficient DC to migrate to lymph nodes for antigen presentation to T cells (
Burns et al. 2001;
de Noronha et al. 2005;
Bouma et al. 2007). At least in murine models, migration defects in WASp-deficient DC impacts initiation of CD4+ and CD8+ adaptive T-cell responses that would be predicted to be biologically significant (
Bouma et al. 2007;
Pulecio et al. 2008). Antigen presentation is further impacted in DC that lack WASp by defective antigen uptake via phagocytosis and impaired formation of an immune synapse with T cells (
Westerberg et al. 2003;
Bouma et al. 2007;
Pulecio et al. 2008). Although most studies have focused on the cross-talk between DC and T cells, cell–cell interaction also occurs between DC and other cell types, and it has recently been shown that NK cell activation is impaired if DC lack WASp (
Catucci et al. 2014). This has potential implications for malignancy in WAS, as NK cells activated by WASp-deficient DC were unable in mouse models to control tumour spread.
The role of pDC in the pathogenesis of WAS has only recently received attention, and to date 2 separate studies have shown that WASp deficiency impacts pDC function, with somewhat different conclusions. In humans, WASp deficiency was reported to result in lower levels of pDC in peripheral blood (
Prete et al. 2013). However, instead of resulting in low levels of Type I IFN, elevated baseline and stimulated levels of IFN-α were observed.
Prete et al. (2013) demonstrated, in a very elegant study, that WASp-deficient pDC are intrinsically hyper-responsive to TLR9 ligands, such as double-stranded DNA because WASp is required for trafficking of TLR9 ligands into the endo-lysosomal compartment in pDC and regulation of TLR9-induced IFN-α responses. This could represent one mechanism for autoimmunity in WAS, where autoantibody/self DNA immune complexes are found at higher levels in the serum and act as a ligand for TLR9 (
Recher et al. 2012;
Prete et al. 2013). A second study also reported low numbers of Type I IFN producing pDC and mDC in mice (
Lang et al. 2013), which was associated with an increased susceptibility to viral infection with lymphocytic choriomeningitis virus (LCMV). Although, at first, this appears incompatible with the observation that WASp deficiency enhances IFN-α responses, murine pDC are chronically activated in vivo, presumably by circulating immune complexes, which then results in exhaustion following additional stimulation (
Prete et al. 2013). Thus it is feasible on the one hand, to have elevated basal levels of IFN-α causing autoimmunity in WAS and on the other hand to have impaired IFN-α up-regulation during infection resulting in viral susceptibility. These data suggest that viral susceptibility in patents with WAS in part relates to lower Type I IFN responses, which could have therapeutic implications.