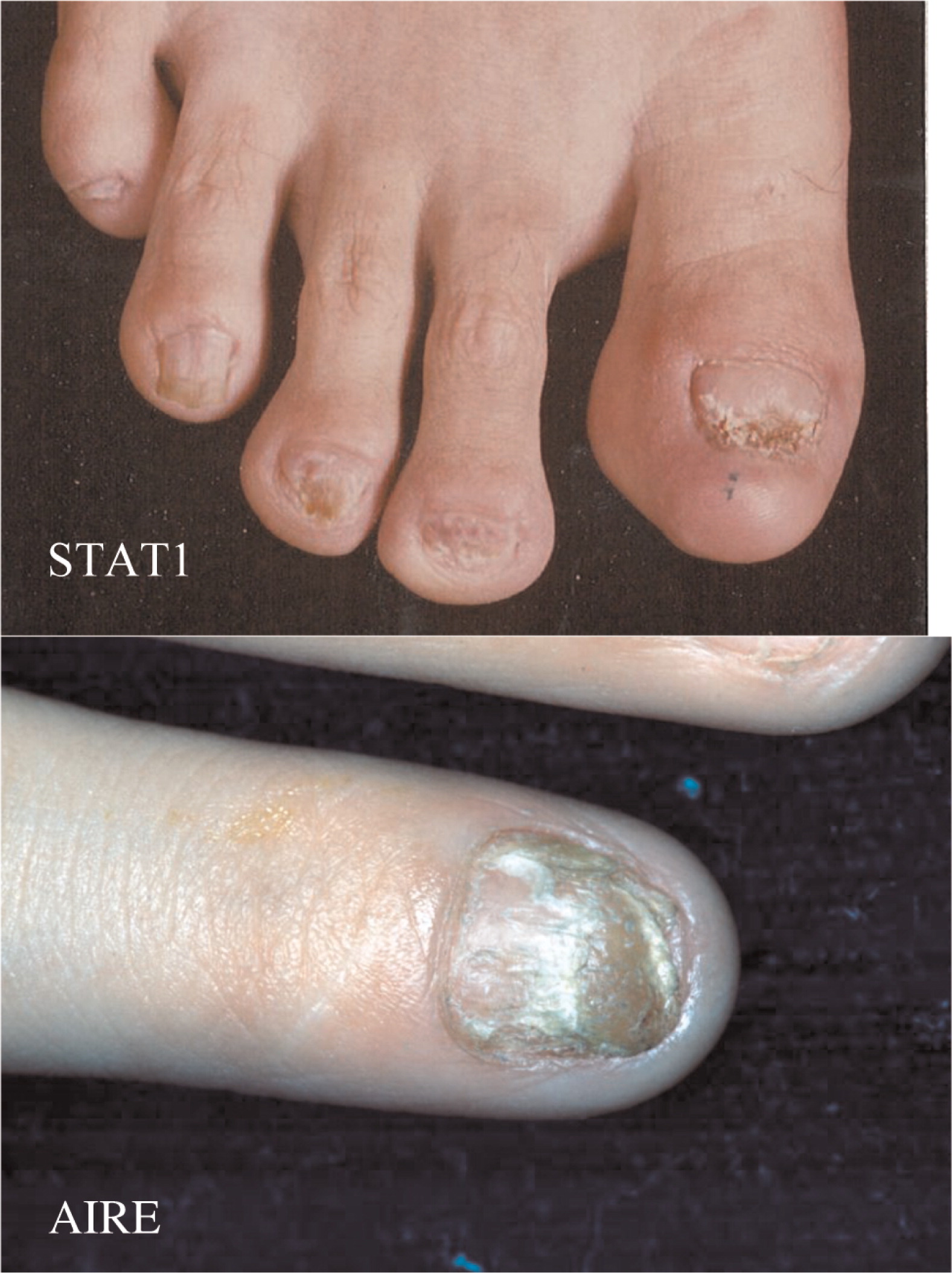
In the past several years it became apparent that mutations in STAT1 are the most common cause of chronic mucocutaneous candidiasis (CMCC) in North America.
The clinical features of thrush and skin and nail lesions are indistinguishable between CMCC caused by an autosomal recessive mutation in the
AIRE gene, which was described 2 decades ago (
Ahonen et al. 1990;
Finnish-German APECED Consortium 1997), or the recently identified monoallelic mutations in
STAT1 (
van de Veerdonk et al. 2011) (
Figure 1). Autoimmune manifestations including endocrinopathies are also shared between these genetic entities. However, it appears that although hypoparathyroidism and hypoadrenodism are more common in
AIRE deficiency, hypothyroidism and diabetes mellitus are more common with
STAT1 mutations. Moreover, recurrent respiratory infections and antibody deficiency have a confirmed association with STAT1 abnormalities, but only rarely in
AIRE deficiency (
Bentur et al. 1991).
Interestingly, clinical entities formerly thought to be distinct, such as autosomal dominant CMCC, are also associated with mutations in
STAT1. The clinical spectrum in these cases is wide (see article by Nahum and Dalal on page 97) ranging from vascular disease in the central nervous system to very mild symptoms of isolated oral thrush. Upon review of all published cases (
Liu et al. 2011;
Maródi et al. 2012;
Takezaki et al. 2012;
Sampaio et al. 2013;
Uzel et al. 2013;
Sharfe et al. 2014), it appears that mutations associated with autosomal dominant CMCC are mostly localized to the coiled–coil domain of
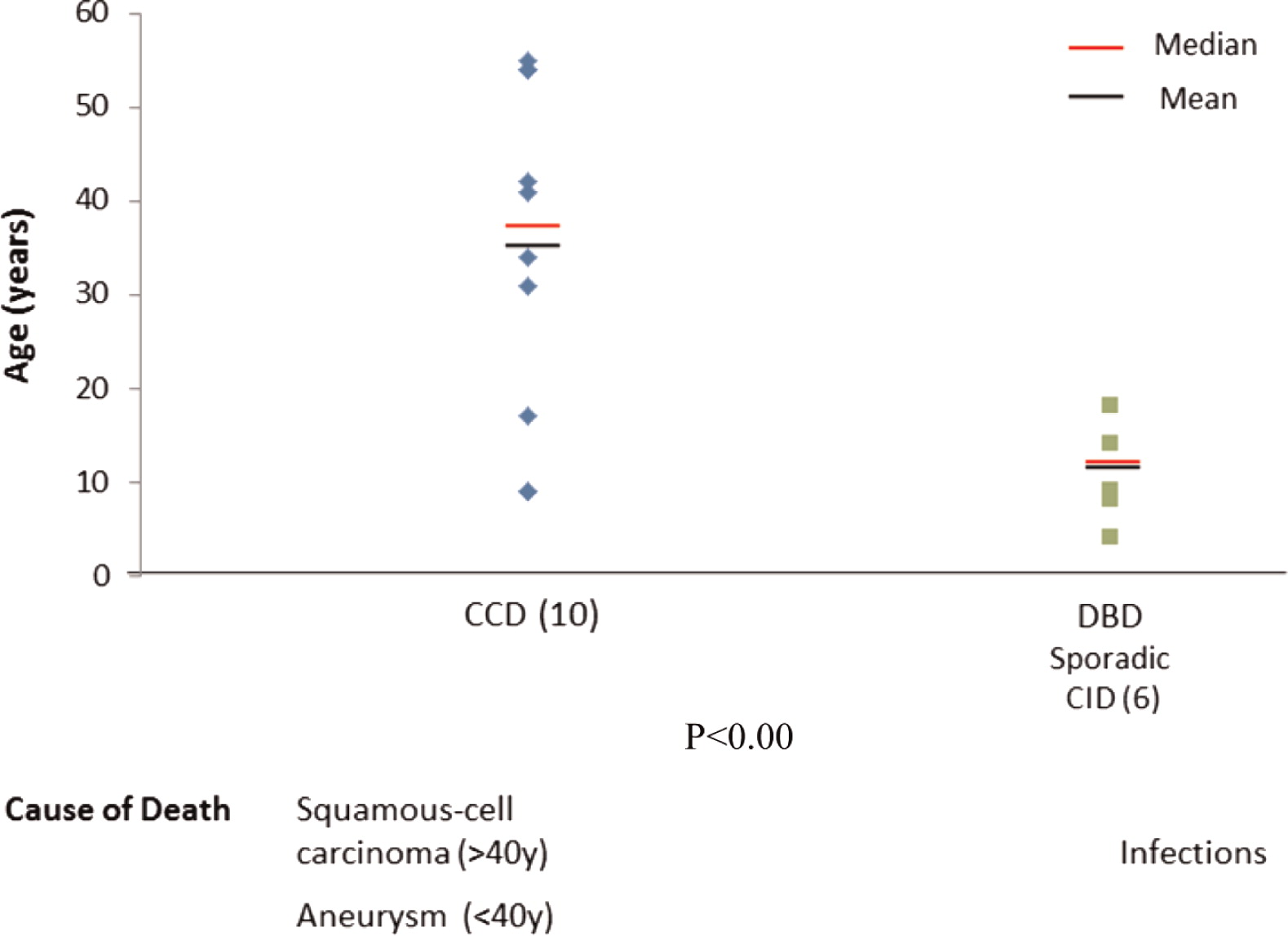
STAT1. The mean and median ages of both of these patients at the time of reporting appears to be around 30 years of age (
Figure 2), but more striking are the mean and median ages of death in this group of patients as young as 35 years and 28 years, respectively (
Figure 3). The cause of death was squamous cell carcinoma in the older patients and stroke in younger individuals.
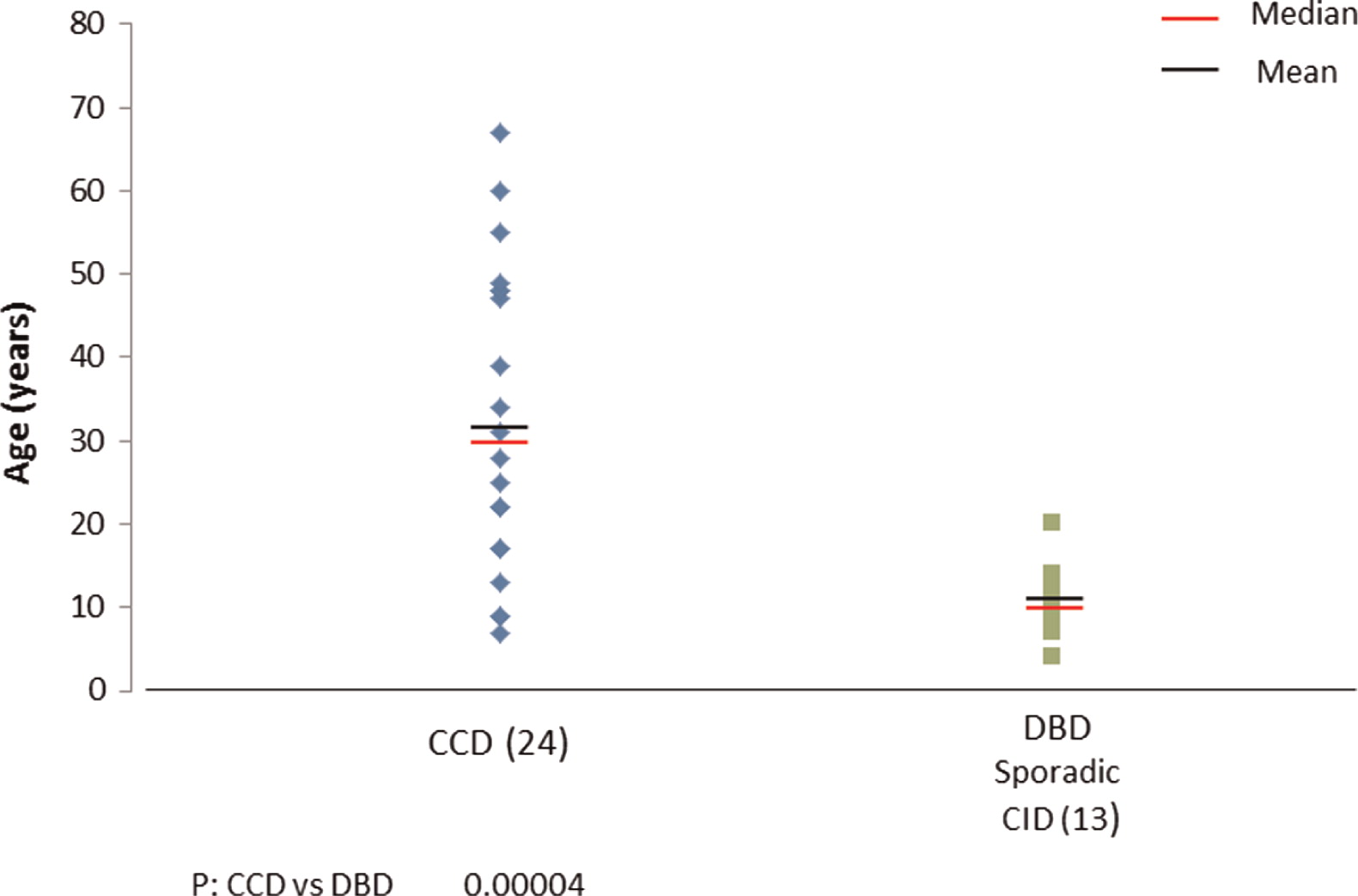
Recently, whole exome sequencing discovered a surprising phenotype of monoallelic
STAT1 deficiency (
Sharfe et al. 2014). Invariably, this group of patients had no family history of immunodeficiency. They present early in life with repeated viral and bacterial infections, and they suffer chronic colitis as well as other autoimmune manifestations. Evaluation of their immune system reveals a progressive decline in immunity, culminating in a combined immunodeficiency including profound lymphopenia and antibody deficiency, practically developing combined immunodeficiency (CID). Most of these patients have mutations in the DNA binding domain (DBD) of
STAT1.
These patients with sporadic CID invariably die of overwhelming viral infection at a mean and median age of 10 years. This analysis, albeit imperfect, suggests clustering of phenotype–genotype correlation.
To date there is no cure for STAT1-related disorders. Although control of bacterial infections can be achieved by using antibiotic therapy and immunoglobulin replacement, other manifestations cannot be prevented or effectively treated. Attempts to clear chronic cytomegalovirus, Epstein–Barr virus, or John Cunningham virus were not successful in these patients. Moreover, vascular changes to the central nervous system, including vasculitis, aneurisms, and acute bleeding cannot be effectively and completely prevented, even with harsh immunosuppressive treatments with cyclophosphoramide. The use of high dose intravenous immunoglobulin failed to show a significant improvement, but the use of immune modulation agents such as Mycophenolate Mofetil may be of benefit in controlling some autoimmune features.
For cases with mutations in the DBD domain, especially those known to be associated with mortality at an early age, such as T385M, hemotopoietic stem cell therapy might be a plausible consideration.