Introduction
The intestine is the largest surface of the body and is constantly exposed to dietary and bacterial antigens as well as potentially noxious substances and infectious agents, which threaten the balance between health and disease (
Salvo Romero et al. 2015). In mammals, the gastrointestinal (GI) tract harbors a complex microbial community, known as the intestinal microbiota, composed of thousands of different species of bacteria, viruses, fungi, archaea, and protists (
Parfrey et al. 2011;
Hillman et al. 2017). This complex community plays a critical role in the digestive process, protection against colonization with pathogens (
Sonnenburg et al. 2004), as well as influencing the maturation and function of the intestinal immune system (
Caricilli et al. 2014). In addition, there is increasing evidence that these microbial communities can regulate brain development, mood, and cognitive function through the bidirectional signaling between the gut and the brain (
Deverman and Patterson 2009;
Lakhan and Kirchgessner 2010). The complexity of the microbiota is matched by the complexity of the host immune system to ensure the maintenance of this balance and for preventing access of organisms to the host inner milieu. Chronic inflammation not only changes the microbiota composition, but it can also trigger significant and long-lasting behavioral changes, such as the development of cognitive impairment and depression, in a bottom-up manner (
Lakhan and Kirchgessner 2010). Taken together, this suggests a critical role for the gut microbiota in regulating brain development and behavior, with the immune system emerging as an important coordinator of these interactions.
Despite the recent discovery of meningeal lymphatics reshaping our understanding of immunity within the central nervous system (CNS) (
Louveau et al. 2015), the brain parenchyma is still considered “immune privileged” compared to peripheral tissues. The brain contains numerous resident immune cells that play a role in the defense against infection and injury, critically supporting neurons, and in maintaining and remodeling circuit connectivity, and regulating plasticity (
Tremblay et al. 2011). Pathways that have been traditionally recognized for their functions in maintaining peripheral immunity are now known to be important in regulating neurodevelopment, including cytokines present in the developing brain, which can regulate neuronal differentiation, axonal growing, and synaptic plasticity (
Deverman and Patterson 2009). In addition, expression of neuroendocrine receptors and factors on immune cells, such as neuropeptide receptors [i.e., calcitonin gene-related peptide (CGRP), substance (SP), adrenomedullin, neurokinins A and B, vasoactive intestinal peptide (VIP), neuropeptide Y (NPY), and gastrin releasing peptide (GRP), etc.], glucocorticoids, or adrenoreceptors can lead to the release of immune-derived mediators and are increasingly recognized for their role in the immune system (
Hadden et al. 1970;
Pert et al. 1985;
Procaccini et al. 2014).
Microbial colonization of the GI tract has a significant impact on neurophysiology and behavior (
Sampson and Mazmanian 2015). Given the immunomodulatory properties of the gut microbiota, peripheral immune cell pathways have been implicated as important mechanisms mediating microbial modulation of brain function and behavior (
Rook et al. 2014;
Rea et al. 2016), including neurodevelopmental disorders, such as autism spectrum disorder (ASD), and GI disease, such as inflammatory bowel disease (IBD), where psychosocial deficits commonly occur in patients. In this review, we discuss the roles for the gut microbiota as an integral mediator of neuroimmune interactions, examining how microbiota interacts between gut and brain-resident immune cells, and how they can impact the etiopathogenesis or manifestation of symptoms relevant to neurobehavioral and neurodegenerative disorders. Finally, we summarize effective probiotic interventions on the function of the CNS and discuss how probiotics can modulate this interaction.
The gut-brain axis
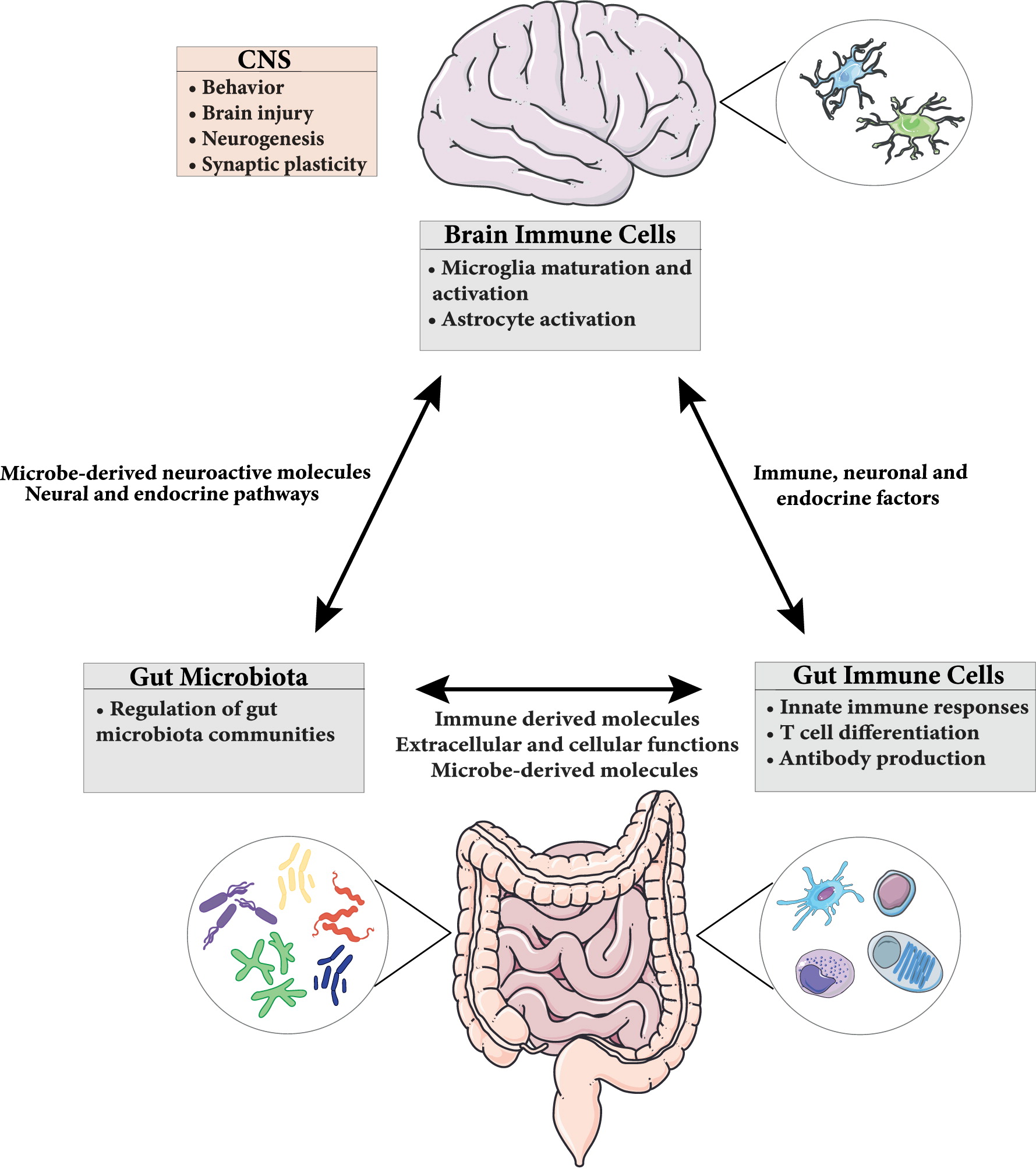
The gut-brain axis plays an important role not only in the proper maintenance of the GI tract but also brain function and behavior. This bidirectional signaling pathway is important in maintaining homeostasis and is regulated at different levels by the CNS, the enteric nervous system (ENS), the hypothalamic pituitary adrenal (HPA) axis, and immune system through complex communication by endocrine, immune, and neuronal factors (
Figure 1) (
Romijn et al. 2008;
Weltens et al. 2018). CNS-mediated regulation of intestinal function and immune responses occurs through systemic, regional, and local pathways. The autonomic nervous system (ANS; sympathetic and parasympathetic branches; drives both afferent and efferent signals), the peripheral nervous system (through the release of neuropeptides), and the HPA axis (through the systemic release of glucocorticoids) are all involved. Moreover, neurally-mediated responses triggered by the activation of sensory afferents in response to changes in luminal content or pressure, muscle distention, and inflammation can be transmitted to target cells through reflexes of local enteric nerves, independent of the CNS (
Carabotti et al. 2015).
In the GI tract, in addition to the well-characterized intestinal epithelial cells (
Sharkey and Mawe 2002) and resident immune cells (
Dantzer 2018), the microbiota also critically contributes to brain-gut communication. This microbiota-gut-brain axis ensures not only the proper maintenance of GI function, but also maintains cognition and emotional behaviors (
Martin and Mayer 2017). Moreover, disturbances of this system have been implicated in a wide range of disorders, including functional and inflammatory GI disorders such as irritable bowel syndrome (IBS) and IBD (
Rhee et al. 2009;
Mayer et al. 2014) as well as neurodevelopmental disorders, such as ASD (
van De Sande et al. 2014).
Physical and biochemical barriers in the GI tract anatomically prevent the microbiota from entering the body and the underlying tissues, restricting it to the lumen. In case of a breach in barriers, immune cells in the GI tract are present to contain and restrict the passage of microbial components, while limiting inappropriate immune activation and overt inflammation (
Hooper et al. 2012), which can impact the microbiota-gut-brain axis.
Probiotics on central nervous system functions
Probiotics are defined as “live micro-organisms which, when administered in adequate amounts, confer a health benefit on the host” (
Morelli and Capurso 2012). While their exact mechanisms of action remain to be completely elucidated, probiotics are considered a novel and relatively safe way (
Yelin et al. 2019) to beneficially modulate the gut microbiota for many GI and extra-intestinal diseases.
Multiple studies have used probiotics to modify the MGB axis, with improvements in behaviors associated with stress-related psychiatric conditions, improved memory abilities, and induction of neuronal plasticity. For instance, probiotic-induced promotion of neurogenesis by increasing hippocampal brain derived neurotropic factor (BDNF) expression normalizes the abnormal response of the HPA axis in mice (
Ait-Belgnaoui et al. 2014).
B. longum,
B. breve,
B. infantis,
L. helveticus,
L. rhamnosus,
L. plantarum, and
L. casei were the most commonly used probiotics for beneficially impacting behaviors, as either single- or multi-strain preparations, all of which were able to improve anxiety, depression, and memory related behaviors, based on multiple animal models (
Wang et al. 2016). For example in depression, each of the single strains of
B. longum,
B. breve,
L. rhamnosus, and
L. helveticus all showed antidepressant effects (
Desbonnet et al. 2008;
Bravo et al. 2011;
Singh et al. 2012;
Ohland et al. 2013). In another study in both rats and mice, these same strains also improved spatial and non-spatial memory abilities (
Luo et al. 2014;
Liang et al. 2015;
Savignac et al. 2015).
In humans, many studies demonstrated a beneficial effect of probiotics on psychiatric conditions in both patients and healthy individuals, although not all studies found a benefit. With respect to anxiety and depression, a probiotic formulation of
B. longum and
L. helveticus could improve anxiety and depression in all participants (
Messaoudi et al. 2011a,
2011b). Other probiotic strains also have effects on anxiety and depression. A probiotic yogurt containing
B. lactis and
L. acidophilus improved general health questionnaire (GHQ) and depression anxiety and stress scale (DASS) scores on petrochemical workers (
Mohammadi et al. 2016) and a recent study using multi-strain probiotics found improvement in the Leiden index of depression sensitivity scale (LEIDS-r score) in healthy participants, demonstrated by a significantly reduced overall cognitive reactivity to sad mood, which is predictive of depression (
Steenbergen et al. 2015).
Similarly, in a study in chronic fatigue syndrome patients,
L. casei Shirota administration decreased anxiety levels following treatment (
Rao et al. 2009;
Ait-Belgnaoui et al. 2014). In contrast, patients with schizophrenia showed no changes in the Positive and Negative Symptom Scale Score (PANSS) after 14 weeks following
L. rhamnosus administration (
Dickerson et al. 2014). Similarly, patients with rheumatoid arthritis showed no change in anxiety levels after intervention with
L. casei for 8 weeks (
Vaghef-Mehrabany et al. 2014). A recent study in healthy volunteers using the Paired Associate Learning test from the Cambridge Neuropsychological Test showed improvements of visuospatial memory performance following intake of
B. longum 1714 (
Allen et al. 2016). Finally, human immunodeficiency virus patients treated with a multi-strain probiotic supplementation (Vivomixx
®; Visbiome
®) also demonstrated improved neurocognitive functions including verbal and visual memory related to memory improvement (
Ceccarelli et al. 2017). Currently, only animal studies have provided strong evidence for a beneficial effect of probiotics on cognition and memory, therefore additional clinical studies are needed to adequately test these effects. The effect of probiotics on the CNS in humans is reviewed extensively elsewhere (
Wang et al. 2016;
Cryan et al. 2019).
Many of these probiotics presumably act beneficially in part by inhibiting the growth of other harmful bacteria or pathogens and (or) improving the immune system (
Luo et al. 2014;
Liang et al. 2015). The proposed mechanisms of actions by which probiotics have a beneficial effect are diverse and comprise endocrine, immune, neural, and metabolic pathways. Many probiotics have shown a reduction in HPA axis activity by decreasing corticosteroid and (or) adrenocorticotropic hormone (ACTH) levels. Our laboratory identified changes in neurogenesis and synaptic plasticity with effects on memory by modulating HPA axis activity. Using a combination of
L. rhamnosus +
L. helveticus, improved memory and cFOS expression, an immediate early gene serving as a neuronal activation marker, was found in mice infected with
C. rodentium following exposure to an acute psychological stress (
Gareau et al. 2011).
Probiotics can also influence the CNS directly via the vagus nerve and by modulating neurotransmitter levels. Treatments that target the vagus nerve increase the vagal tone and inhibit cytokine production. The stimulation of vagal afferent fibers in the gut influences monoaminergic brain systems within the brain stem that play crucial roles in major psychiatric conditions, such as mood and anxiety disorders. For example, behavioral changes following
L. rhamnosus and
B. longum administration, were not seen in vagotomized animals (
Bercik et al. 2011b). Furthermore,
B. fragilis could enhance serum tryptophan levels, a precursor of 5-hydroxytryptamine (5-HT) or serotonin synthesis (
Emge et al. 2016). Serotonin is an important neurotransmitter in the gut that can stimulate peristalsis and induce nausea and vomiting by activating the vagus nerve. Interactions between the vagus nerve and serotonin systems, with the gut microbiota controlling tryptophan catabolism, appear to play an important role in the treatment of psychiatric conditions (
Breit et al. 2018).
Finally, another mechanism of action of probiotics altering CNS function is thought to be by decreasing pro-inflammatory cytokines and increasing anti-inflammatory cytokines. Cultured intestinal mucosal tissues of CD patients with
L. casei,
L. bulgaricus,
L. crispatus and
E. coli showed an interaction with immunocompetent cells and modulation of the production of pro-inflammatory cytokines, resulting in a significant reduction in the pro-inflammatory cytokine TNF-α (
Borruel et al. 2002). In IL-10-deficient mice, which spontaneously develop colitis, significant reductions of INF-γ and TNF-α by Peyer’s patch lymphocytes and pro-inflammatory cytokine production by splenocytes were found in probiotic-treated mice (
McCarthy et al. 2003). In healthy rats, the administration of a mixture of Lactobacillus and Bifidobacterium upregulated IL-10 and downregulated TNF-α and IL-6 (
Karamese et al. 2016). Moreover, probiotics can ameliorate inflammatory immune responses through modulation of the intestinal barrier permeability (
Llopis et al. 2005,
2009;
Rao and Samak 2013). Increased intestinal barrier permeability is associated with psychiatric disorders, such as depression and ASD, while it can be restored by probiotic formulations of
B. longum and
L. helveticus, along with improved CNS function (
Arseneault-Breard et al. 2012;
Hsiao et al. 2013).
It is important to highlight the limitations of probiotics studies in both animals and in humans. With respect to the translation of behavioral models in animals to human health, it is important to note that the tests used to measure behavior in animals have no direct equivalents in humans. Theses behavioral tests are designed to reflect the presumed CNS dysfunction in humans, based on specific animal behaviors. The common use of questionnaires in humans to assess behaviors could result in subjective biases. In the future, use of neuroimaging techniques may provide a better, more accurate, alternative way to assess changes in brain function (
McFarland et al. 2018). Several studies have found that in contrast to inbred mice, humans present individual, regional, and strain-specific mucosal bacterial colonization patterns. In some individuals, these unique features prevents standard probiotics from transiently colonizing the gut, as determined by indistinguishable probiotic presence in the stool (
Zmora et al. 2018). In the absence of individual-specific mucosal colonization to probiotics, lack of beneficial effects could be misunderstood (
Llopis et al. 2009). Of importance for future studies will be the development of personalized medicine using strain-specific and individual-specific based probiotic therapy based on next-generation sequencing, to enable persistent live-bacteria colonization to beneficially impact the host.
Conclusions
Accumulating evidence indicates that brain-resident and gut-resident immune cells are critically involved in orchestrating MGB axis communication. The gut microbiota can influence the development and function of the intestinal immune system and conversely, the innate and adaptive immune system influence microbiota composition. This reciprocal relationship contributes to establishment of symbiosis with the gut microbiota, preventing potentially harmful bacteria and pathogens from breaching the host defenses. The microbiota can also modulate brain microglia and astrocytes, which mediate neurophysiological processes including neural development, neurotransmission, and CNS immune activation. These microbial influences on immune responses have important consequences for brain inflammation, injury, behavior and brain plasticity and are increasingly associated with symptoms of various neuroinflammatory and psychiatric disorders. This relationship establishes a tight communication with the CNS and immune system as important cellular mediators across the gut-brain axis. Disruption of these complex and dynamic interactions can have profound consequences for host health.
Studies of probiotics in animal models, and a few studies in humans, have identified their effective application in the prevention and treatment of various health conditions and diseases such as GI infections but also in improving CNS function, including psychiatric disorders such as anxiety and depression, and cognition.
Finally, as the specific pathways of the MGB axis signaling are still incompletely characterized, it is critical for future studies to clarify these pathways to define specific microbe-derived factors, immune effector functions, and microbiota–immune pathways for modulating brain function and behavior. Also, it is crucial for future microbiota studies that appropriate controls be used to compare baseline variation in experimental groups. Likewise, it is important to note that laboratory mice have distinct immunological profiles from humans, limiting the ability to directly extrapolate conclusions to human diseases. Future mechanistic insights into how the immune system and microbiota interact will require development of novel approaches with high throughput analysis of metabolites as well as gene and protein expression, to move towards a personalized-based therapy.