Introduction
The epidemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing coronavirus disease 2019 (COVID-19), has rapidly spread worldwide. In adults, COVID-19 is typically characterized by severe interstitial pneumonia and hyperactivation of the inflammatory cascade. In children, the respiratory involvement appears to have a more benign course, with almost no fatalities reported in this age group (
Verdoni et al. 2020). However, a few months into the pandemic, reports from the United Kingdom documented a presumed post-infectious immune-mediated condition in children with features similar to Kawasaki disease (KD) (
Riphagen et al. 2020). This condition, a new disease in children, has been termed multisystem inflammatory syndrome in children (MIS-C); also referred to as pediatric multisystem inflammatory syndrome (PMIS). While the incidence of this condition is yet uncertain, it appears to be a relatively rare complication of COVID-19 in children, probably occurring in less than 1% of children with confirmed infection.
Dufort et al. (2020) reported an estimated incidence of laboratory-confirmed infection in individuals <21 yr old of 322 per 100 000, with the incidence of MIS-C occurring in 2 per 100 000. Recent reports show approximately 4000 cases of MIS-C in the United States and 35 MIS-C associated deaths (
DeBiasi 2021). The criteria for diagnosis of MIS-C were defined by several organizations, including the Centers for Disease Control and Prevention (CDC) and World Health Organization (WHO) (guidelines are available on each organizations’ website). While the core of these criteria is similar, some differences are important. The CDC mandated only one day of fever or a subjective report of fever lasting 24 h, while the WHO determined 3 days of fever as an essential criteria. Both require multisystem involvement of at least 2 different organ systems and support of a positive laboratory marker. While the CDC requires a positive test for COVID-19 (PCR, serology, antigen), or exposure within 4 wk prior to onset, the WHO criteria requires evidence of contact with an individual with COVID-19.
MIS-C usually presents with fever and multiorgan involvement, with elevated inflammatory markers weeks after exposure to SARS-CoV-2 (
Verdoni et al. 2020;
Whittaker et al. 2020). Cardiac involvement is very common and may include arrhythmias, depressed myocardial function, valvular regurgitation, or coronary artery inflammation (
Valverde et al. 2021). MIS-C bares resemblance to KD, a rare acute vasculitis, presenting with persistent fever, exanthema, lymphadenopathy, conjunctival injection, changes to the mucosae and extremities, with coronary artery aneurysms being the main complication (
Verdoni et al. 2020). These conditions quite often share several features and up to 50% of children with MIS-C fulfill the criteria for complete or incomplete KD (
Dufort et al. 2020;
Verdoni et al. 2020;
Whittaker et al. 2020). Notably, there are some key distinctive features: MIS-C commonly affects older children and adolescents, while KD affects younger children; gastrointestinal symptoms are very common in MIS-C and less prominent in classic KD. Cardiovascular features are also different; myocardial dysfunction and shock are far more common in MIS-C, while the risk of coronary artery involvement in MIS-C is unclear in comparison to classic KD. Ultimately, the designation is based on COVID-19 serology testing or exposure history—patients with positive serologic test (or with an exposure to an individual with COVID-19 infection) who also fulfill criteria for complete or incomplete KD are considered to have MIS-C (
Verdoni et al. 2020;
DeBiasi 2021).
Patient descriptions
We present two unique patients with an unusual clinical presentation, from both sides of the pediatric range of ages; the first is a 4-mo-old infant and the second is a 16-yr-old teenager.
Patients and/or legal guardian consented to the publication of the presented data.
The first case is a male 4-mo-old infant who was otherwise healthy. He was admitted to our department due to prolonged fever, without an apparent infectious cause, and without response to empirical antibiotic treatment. The patient presented to the pediatric emergency department due to continuation of fever for over 10 days, accompanied by cough and rhinorrhea, and following 8 days of antibiotic therapy. No additional complaints were noticed by the parents, and no recent contact with febrile individuals was known, however, careful history taking revealed that the mother was ill with COVID-19 while pregnant a few weeks prior to delivery. The diagnosis of COVID-19 was verified at the time by a positive PCR test. Upon arrival to delivery room, the mother was again tested and found COVID-19 negative by PCR testing. On presentation, the patient was febrile and his other vital signs were within normal range. A physical examination revealed fissured lips, slight bilateral conjunctivitis, and a mild bilateral and symmetrical rash involving the lower limbs.
Laboratory workup showed slight anemia with hemoglobin of 9.6 gr/dL (normal range 11.1–14.1 gr/dL), leukocytosis of 23.5 × 103/μL (normal range 6.0–17.5 × 103/μL), with neutrophilia of 13.6 × 103/μL (normal range 1.0–8.5 × 103/μL) and monocytosis of 1.9 × 103/μL (normal range 0.16–1.0 × 103/μL), thrombocytosis of 534 × 103/μL (normal range 150–350 × 103/μL), and elevated C-Reactive Protein at 7.95 mg/dL (normal range 0.02–0.5 mg/dL). Nasal swab PCR for a panel of respiratory viruses, including COVID-19, was obtained and found negative. In addition, serology tests for Epstein Barr virus (EBV) and cytomegalovirus (CMV) were obtained and demonstrated only previous infection (most probably maternal antibodies). Due to a PCR verified maternal COVID-19 disease during pregnancy, serology for COVID-19 was also obtained and found negative. As part of the routine workup, echocardiogram was preformed demonstrating coronary artery wall dilation. The right coronary artery was 3–4 mm matching a Z score of 4.7, proximal and distal left coronary artery was 3 mm with Z score 5–6, with pericardial fluid and preserved cardiac function. Therefore, due to in utero exposure to COVID-19, an inflammatory disease such as MIS-C or incomplete KD was a probable etiology and treatment with intravenous immunoglobulins (IVIG), corticosteroids and aspirin was given, resulting in resolution of fever, conjunctivitis and rash. On a follow up visit, it appeared that the coronary arteries did not dilate or deteriorate further, there were no signs of heart failure, and the infant was vital and active. Subsequent follow up visits revealed no further dilation of the coronaries.
The second patient is a 16-yr-old previously healthy female, who was admitted to our department due to recurrent brief episodes of chest pain, starting 14 days before hospitalization. She came to the pediatric emergency department twice in 10 days complaining of chest pain and tightness lasting a few seconds and resolving spontaneously, radiating to both shoulders and accompanied by dyspnea. She denied recent febrile episodes or any other complaints. On her first visit to the emergency department, the patient’s vital signs and physical examination were within normal limits; ECG, chest X-ray and troponin levels were all normal, and she was therefore discharged. The patient returned to hospital due to continuation of symptoms. She was hemodynamically and respiratory stable, and physical examination was within normal range. Laboratory workup showed hemoglobin of 11.3 gr/dL (normal range 12–16 gr/dL), white blood cell count of 13.27 × 103/μL (normal range 4.5–13.5 × 103/μL), with neutrophilia of 10.95 × 103/μL (normal range 1.9–8 × 103/μL), thrombocytosis of 605 × 103/μL (normal range 150–350 × 103/μL), and elevated C-Reactive Protein at 17.2 mg/dL (normal range 0.02–0.5 mg/dL). PCR swab for respiratory viruses including COVID-19 was negative.
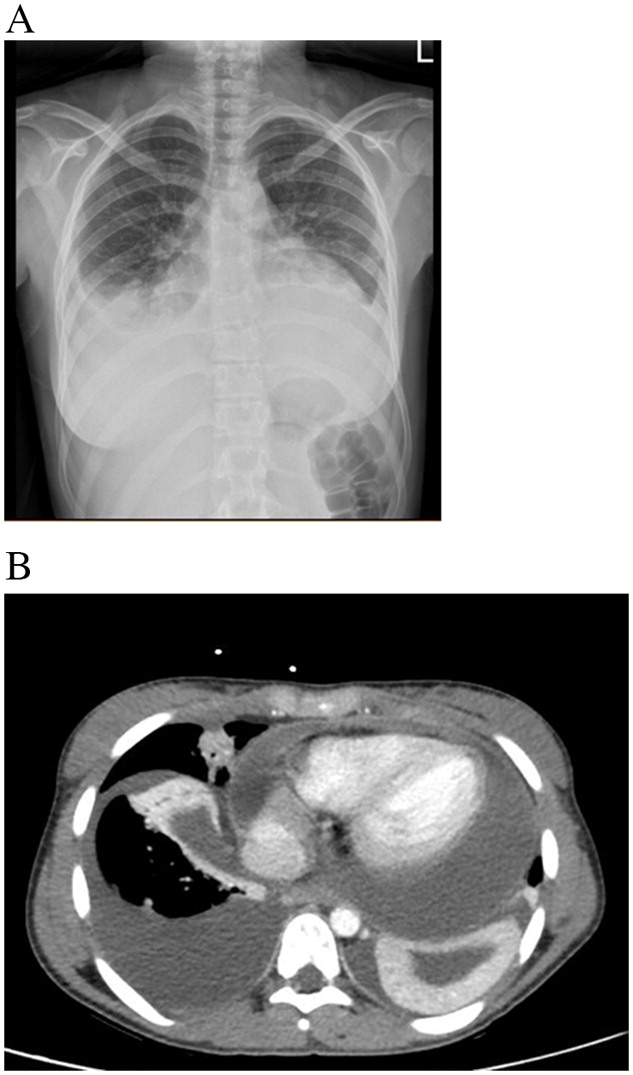
By the end of first day of hospitalization a rapid deterioration was noticed–the patient was dyspneic with desaturation, tachycardia and fever of 38.4 °C. A physical examination revealed diminished breath sounds, and hepatomegaly, without peripheral edema. Repeated laboratory assessment showed troponin <13 ng/dL (normal range 0–14 ng/dL), elevated fibrinogen levels of 838 mg/dL (normal range 200–500 mg/dL), and D-DIMER of 8475 ng/mL (normal range 0–500 ng/mL). ANA, IgG levels, and ASLO were all within normal limits. At this time a serologic test for COVID-19 was taken and found to be positive, demonstrating recent non-symptomatic infection. A second chest X-ray showed bilateral pleural effusion and possibly a wide cardiac silhouette (
Figure 1A), and on ECG sinus tachycardia with low QRS voltage. An echocardiogram and a CT scan were performed, confirming a moderate pleural effusion and demonstrating a massive pericardial effusion causing tamponade mandating pericardiocentesis (
Figure 1B). CT guided drainage of the pericardial fluid yielded one liter of pericardial fluid. Treatment with high dose steroids as well as colchicine was administered with gradual clinical and laboratory improvement. Blood and pericardial fluid cultures were all found to be negative. PCR for a wide panel of viruses (Coxsackievirus, echovirus, adenovirus, EBV, CMV, influenza, etc.) was negative as well. We further tested the pericardial fluid with a RT-PCR-based pan-bacterial marker (16S), qPCR fungal panel, mycobacteria culture, and other PCR assays which were all found negative.
Repeated chest X-ray during hospitalization demonstrated improvement in pleural effusion, and echocardiogram showed hyperemia of pericardium with preserved left ventricular function and size, without signs of constrictive pericarditis.
Discussion
Over the past year, we have observed the intriguing and many faces of the COVID-19 epidemic. Whereas in the adult population the manifestations are mainly acute respiratory and sometimes multisystem in nature, which in many cases is fatal, the pediatric population are inflicted by a post-infectious manifestation, MIS-C, which overlap in certain aspects with KD.
MIS-C appears as a spectrum of severity and with various manifestations, with most patients having prolonged fever and elevation of inflammatory markers. Many present with abdominal pain, and quite often, cardiac involvement ranging from a mild reduction in cardiac function to the more alarming cardiovascular shock. Symptoms usually encompass other organs as well, including the skin and CNS (
Dufort et al. 2020;
Riphagen et al. 2020;
Verdoni et al. 2020).
In most case series affected children were above 5 yr of age, however, younger patients with the disease have also been described. MIS-C has not been well described in young infants and neonates. The first case presented here suggests the possibility that prenatal, in utero, exposure to COVID-19 may later cause MIS-C. The fact that the patient’s mother and immediate family were extremely ill in the weeks prior to delivery supports this possibility. The pathophysiological mechanism underlying such a phenomenon is yet unknown. One possible mechanism is that maternal infection with SARS-CoV-2 results in the development of IgG antibodies against a viral antigen that can cross the placenta. This was shown in a recent study that described 6 mothers with confirmed COVID-19 infection, wherein high concentrations of virus-specific IgG in the blood samples of 5 neonates was detected, despite PCR nasal swabs being negative (
Zeng et al. 2020)
. Placental passage of protective IgG maternal antibodies can provide passive immunity to the newborn but might also trigger production of autoantibodies, later causing activation and secretion of pro-inflammatory cytokines that result in development of MIS-C (
Gray et al. 2021;
Pawar et al. 2021). This suggested mechanism is akin to neonatal lupus, where maternal anti-SSA and anti-SSB antibodies may cause manifestations such as rash and congenital heart block in newborns. IgG producing antibody secreting cells (ASC) have been known to increase significantly during the acute stage of KD and decrease after IVIg administration, indicating their involvement in inflammation associated with KD (
Xu et al. 2019). This suggests the possible contribution of autoantibodies or antibodies to SARS-CoV-2 in the pathogenesis of MIS-C, as is presumed in KD (
Kabeerdoss et al. 2021). One interesting report described a case of known COVID-19 maternal infection with elevated IgM antibody levels in her newborn, detected 2 h after birth. This suggests that the neonate was infected in utero, since IgM antibodies are not transferred to the fetus via the placenta (
Dong et al. 2020). These represent antibodies produced in utero, and while at this stage of development the adaptive immune system is far from being mature, it is possible that certain antigens may cause such a phenomenon. In the case of the infant presented here, we postulate that he could have been COVID-19 positive but asymptomatic after birth, and only later developed the post-infectious complications of MIS-C. The presence of antibodies in infant serum is likely related to the timing of maternal disease, and the time required to produce antibodies before transferring them to the embryo.
Intrapartum transmission due to exposure to maternal infected secretions and feces may be a possibility (
Sankaran et al. 2021), however, is unlikely as the mother was not acutely ill during labor. Another potential explanation is development of fetal inflammatory response syndrome (FIRS), which is characterized by systemic inflammation and an elevation of fetal plasma interleukin-6, and observed in some cases due to fetal viral infections such as CMV (
Gotsch et al. 2007). Therefore, FIRS stimulated by maternal viral load should be considered even in the absence of vertical transmission (
McCarty et al. 2021). This theory is less relevant in our current case, in which the infant had been well through 3 uneventful months before presentation.
Reports of this disease affecting neonates has led to the new term of neonatal multisystem inflammatory syndrome (MIS-N), defined as MIS-C in the first 2 wk of life (
Zeng et al. 2020).
Since the distinction of KD versus MIS-C is based ultimately on known exposure to SARS-Cov-2 infection, we thus diagnosed the patient with MIS-C and treated him accordingly with steroids, in conjunction with the traditional KD regimen of IVIG and aspirin.
The second case reported here is unique due to the clinical presentation of a massive pericardial effusion, causing cardiac tamponade, without affecting the actual myocardial tissue, e.g., lack of elevated BNP or troponin. While pericardial effusion was reported in up to 30% of affected subjects at administration in a recent European study (
Valverde et al. 2021), it is frequently associated with various degrees of myocardial damage and elevated cardiac bio-markers. Moreover, depressed left ventricular ejection fraction (LVEF) is a common presentation reported in up to 60% of patients, and, abnormalities in myocardial systolic and diastolic function were present even in MIS-C patients with preserved EF (
Dufort et al. 2020;
Feldstein et al. 2020;
Whittaker et al. 2020;
Alsaied et al. 2021). A review comparing control subjects and patients with KD to a MIS-C group showed MIS-C patients had lower LVEF and reduced measures of global LV systolic strain and strain rate (
Alsaied et al. 2021). To date, recovery of systolic function has been characterized only in small retrospective case series (
Belhadjer et al. 2020;
Dufort et al. 2020;
Feldstein et al. 2020;
Riphagen et al. 2020;
Whittaker et al. 2020;
Alsaied et al. 2021), but early reports suggest LVEF normalizes in most patients within 1 to 2 wk after initial presentation (
Belhadjer et al. 2020). In our patient the clinical picture was a massive pericardial fluid causing life threatening cardiac tamponade. Once drained, the patient recovered quickly and without any cardiac or other sequelae.
These two unique cases encountered in a post COVID-19 infection scenario illustrate the complexity and fascinating daily questions encountered by physicians around the world. We believe it is of great importance to study and share these cases since the understanding of a new disease is crucial for early diagnosis and establishing the appropriate treatment modalities.