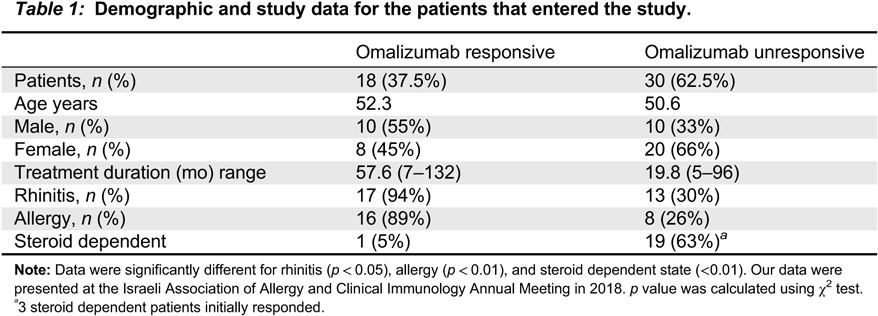
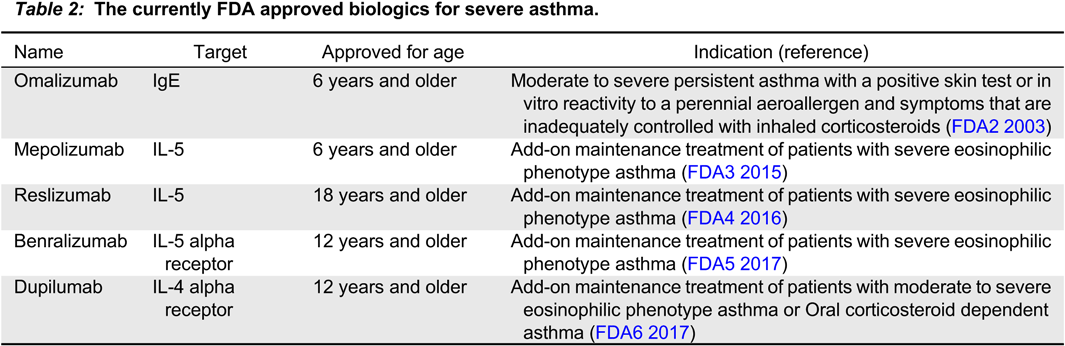
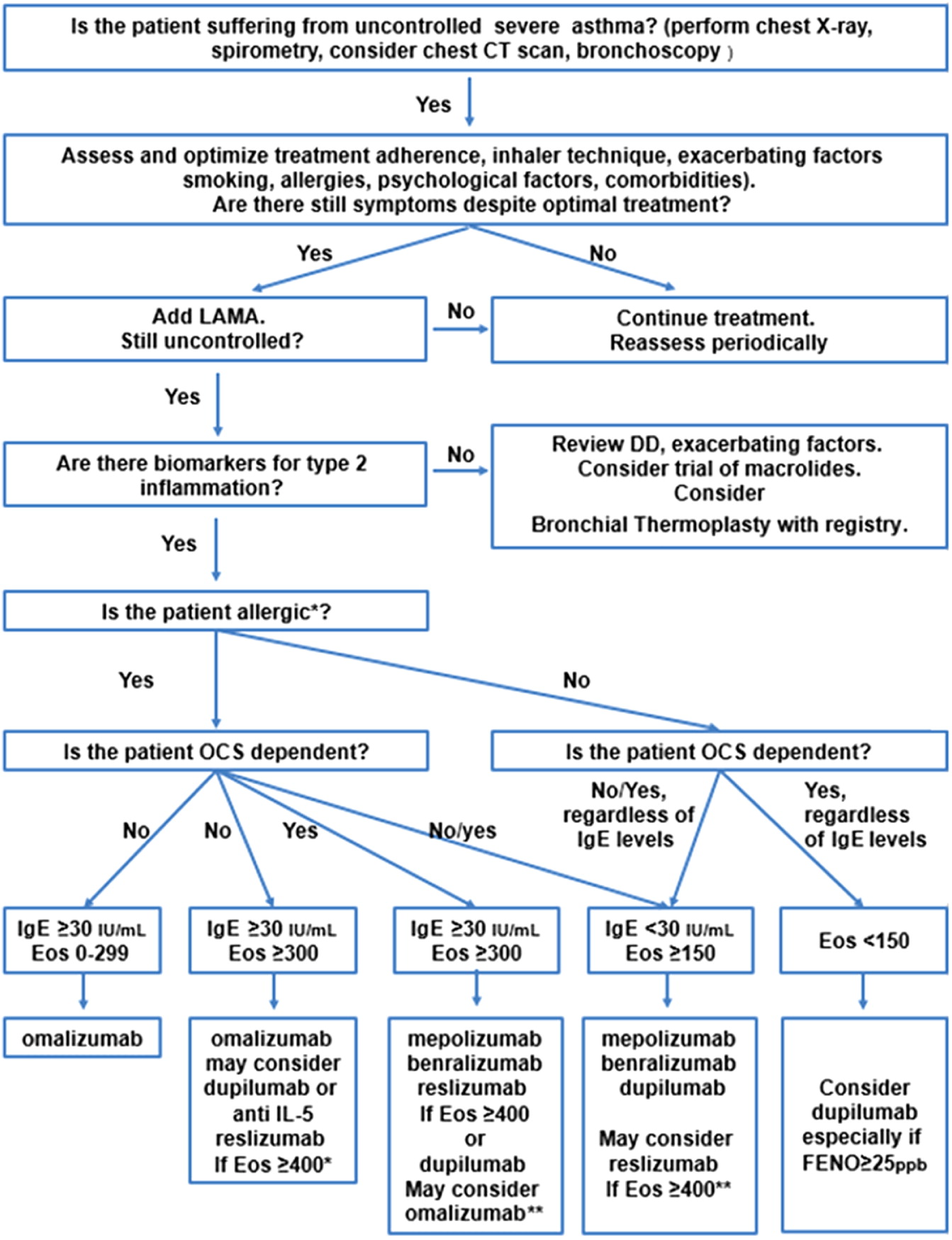
Lefaudeux D., De Meulder B., Loza M.J., Peffer N., Rowe A., Baribaud F., Bansal A.T., Lutter R., Sousa A.R., Corfield J., Pandis I., Bakke P.S., Caruso M., Chanez P., Dahlén S.E., Fleming L.J., Fowler S.J., Horvath I., Krug N., Montuschi P., Sanak M., Sandstrom T., Shaw D.E., Singer F., Sterk P.J., Roberts G., Adcock I.M., Djukanovic R., Auffray C., Chung K.F., Adriaens N., Ahmed H., Aliprantis A., Alving K., Badorek P., Balgoma D., Barber C., Bautmans A., Behndig A.F., Bel E., Beleta J., Berglind A., Berton A., Bigler J., Bisgaard H., Bochenek G., Boedigheimer M.J., Bøonnelykke K., Brandsma J., Braun A., Brinkman P., Burg D., Campagna D., Carayannopoulos L., Carvalho da Purfição Rocha J.P., Chaiboonchoe A., Chaleckis R., Coleman C., Compton C., D’Amico A., Dahlén B., De Alba J., de Boer P., De Lepeleire I., Dekker T., Delin I., Dennison P., Dijkhuis A., Draper A., Edwards J., Emma R., Ericsson M., Erpenbeck V., Erzen D., Faulenbach C., Fichtner K., Fitch N., Flood B., Frey U., Gahlemann M., Galffy G., Gallart H., Garret T., Geiser T., Gent J., Gerhardsson de Verdier M., Gibeon D., Gomez C., Gove K., Gozzard N., Guo Y.K., Hashimoto S., Haughney J., Hedlin G., Hekking P.P., Henriksson E., Hewitt L., Higgenbottam T., Hoda U., Hohlfeld J., Holweg C., Howarth P., Hu R., Hu S., Hu X., Hudson V., James A.J., Kamphuis J., Kennington E.J., Kerry D., Klüglich M., Knobel H., Knowles R., Knox A., Kolmert J., Konradsen J., Kots M., Krueger L., Kuo S., Kupczyk M., Lambrecht B., Lantz A.S., Larsson L., Lazarinis N., Lone-Satif S., Marouzet L., Martin J., Masefield S., Mathon C., Matthews J.G., Mazein A., Meah S., Maiser A., Menzies-Gow A., Metcalf L., Middelveld R., Mikus M., Miralpeix M., Monk P., Mores N., Murray C.S., Musial J., Myles D., Naz S., Nething K., Nicholas B., Nihlen U., Nilsson P., Nordlund B., Östling J., Pacino A., Pahus L., Palkonnen S., Pavlidis S., Pennazza G., Petrén A., Pink S., Postle A., Powel P., Rahman-Amin M., Rao N., Ravanetti L., Ray E., Reinke S., Reynolds L., Riemann K., Riley J., Robberechts M., Roberts A., Rossios C., Russell K., Rutgers M., Santini G., Sentoninco M., Schoelch C., Schofield J.P.R., Seibold W., Sigmund R., Sjödin M., Skipp P.J., Smids B., Smith C., Smith J., Smith K.M., Söderman P., Sogbesan A., Staykova D., Strandberg K., Sun K., Supple D., Szentkereszty M., Tamasi L., Tariq K., Thörngren J.O., Thornton B., Thorsen J., Valente S., van Aalderenm W., van de Pol M., van Drunen K., van Geest M., Versnel J., Vestbo J., Vink A., Vissing N., von Garnier C., Wagerner A., Wagers S., Wald F., Walker S., Ward J., Weiszhart Z., Wetzel K., Wheelock C.E., Wiegman C., Williams S., Wilson S.J., Woosdcock A., Yang X., Yeyashingham E., Yu W., Zetterquist W., and Zwinderman K. 2017. U-BIOPRED clinical adult asthma clusters linked to a subset of sputum omics. J. Allergy Clin. Immunol. 139:1797–1807.