Patient 1 was born to non-consanguineous parents of French–Irish and Native American origin. At 30 weeks of gestation, an ultrasound revealed small long bones and a bell-shaped chest. The male patient was born at term with a weight of 3.4 kg. The patient was noted to have bowed legs, fine sparse hair, and constipation with no evidence of Hirschsprung disease. Genetic analysis demonstrated homozygosity for the common 70A>G mutation in the
RMRP gene. The patient suffered from recurrent respiratory infections that were often treated with antibiotics. Immune assessment, first performed at 2 years of age, demonstrated appropriate immunoglobulin levels and production of antibodies to tetanus vaccine (1.38 IU/mL) and EBV. However the number of T cells and the response to PHA were reduced (
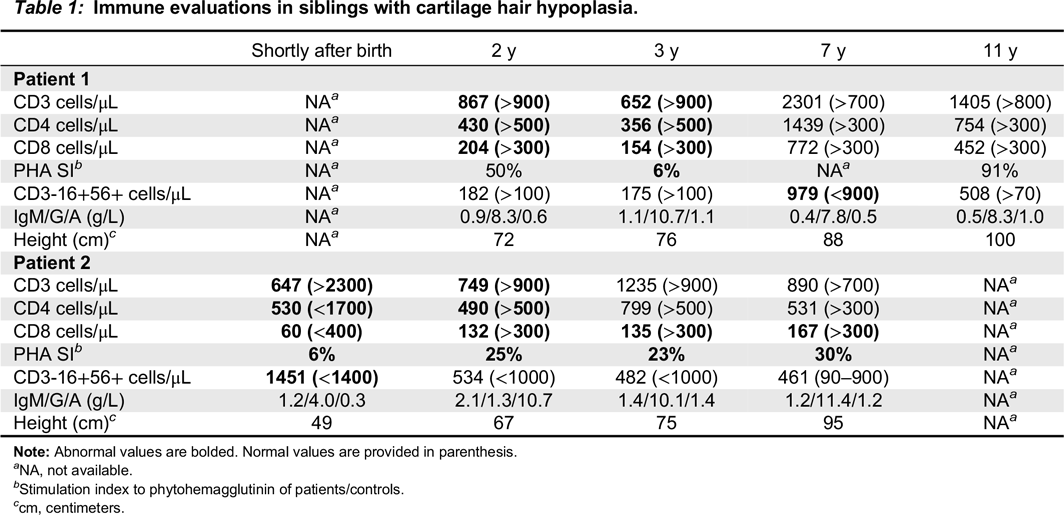
Table 1). Follow-up evaluations indicated further deterioration in T cell numbers and responses. By 4 years of age, analysis of the T cell repertoire, performed by flow cytometry as previously described (
Grunebaum et al. 2006), demonstrated impaired diversity of CD4+ T cells (not shown) and CD8
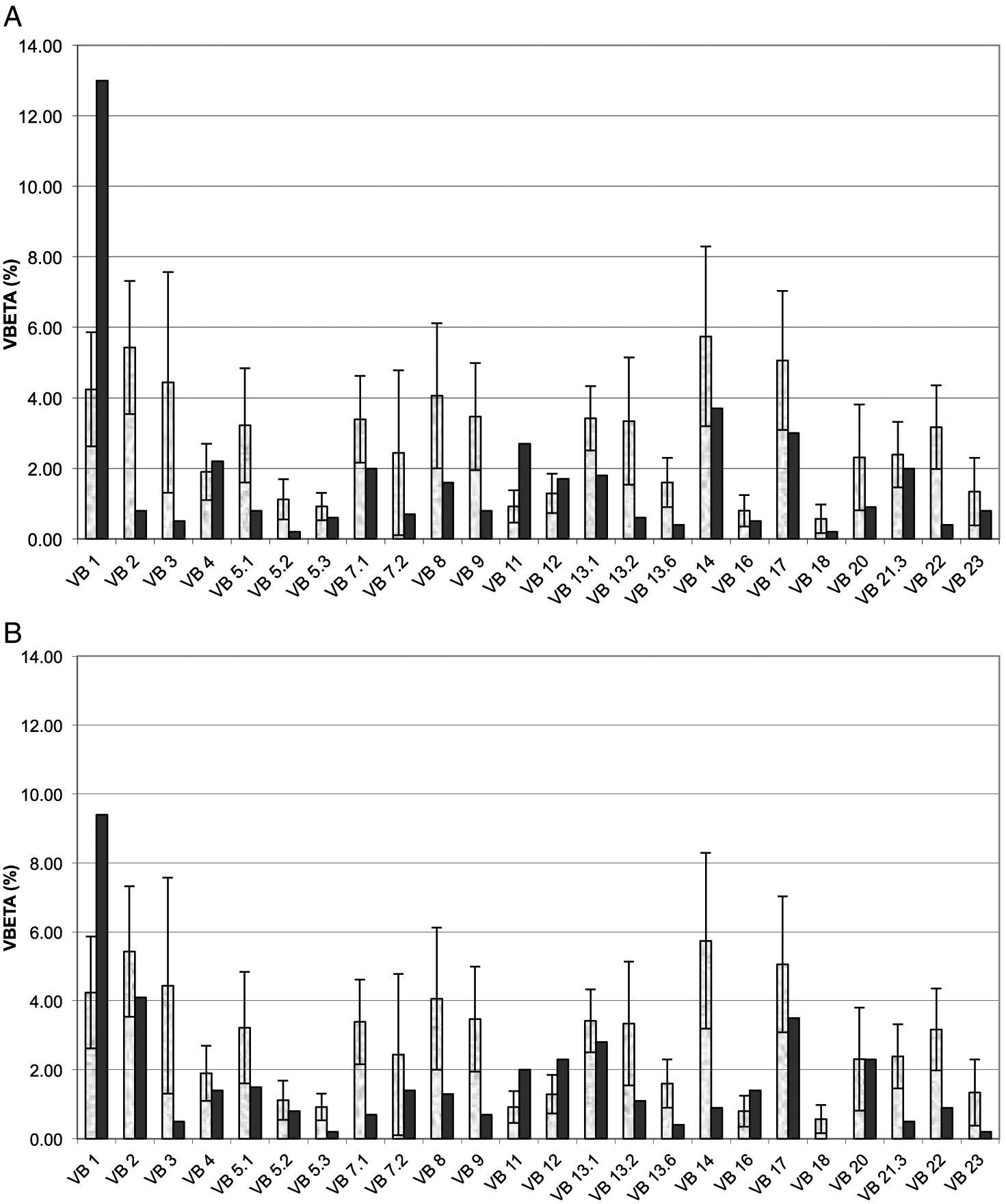
+ T cells (
Figure 1A), with expansion of some families and underrepresentation of others. An HLA-identical sibling was identified and following extensive discussions with the family, the patient underwent HSCT at 4.5 years of age. HSCT was performed with busulfan (16 mg/kg) and cyclophosphamide (200 mg/kg) conditioning as well as prednisone and cyclosporine for GvHD prophylaxis, as previously described (
Grunebaum et al. 2006). The patient suffered grade 1 acute GvHD of the skin that resolved with a short course of prednisone. One year after HSCT, the patient developed skin rash. Skin biopsy demonstrated grade II acute GvHD with some features of chronic or healing GvHD. No other features of GvHD were observed. The patient was treated with prednisone with rapid response followed by a course of physiotherapy and methotrexate that was discontinued 5 years after HSCT. The patient continued to suffer from residual mild wrists and ankles joint contractures as well as mild scleroderma-like skin changes and tightness. Repeated evaluations, as late as 7 years after HSCT, demonstrated complete donor engraftment and immune reconstitution with normalization of T cell numbers and diversity, as well as response of T cells to stimulation and production of antibodies. The patient’s height at 11 years of age (
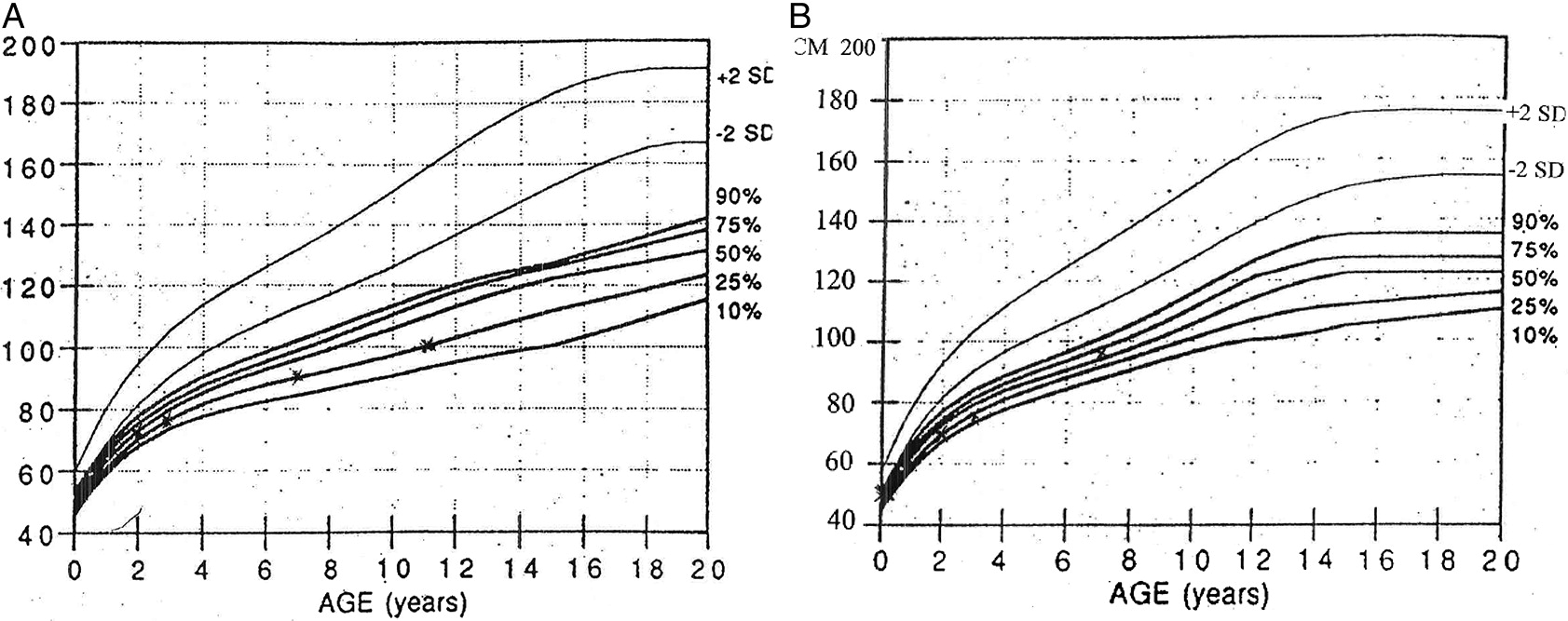
Figure 2A), plotted on growth charts for CHH (
Mäkitie et al. 1992) has remained on the 15
th percentile.
Patient 2, a female, was born 4 years after patient 1. Antenatal ultrasound showed short limbs and genetic analysis confirmed the homozygous
RMRP mutations. At 10 days of age the patient suffered from respiratory syncytial virus pneumonia, requiring a 2-week admission for supportive care. In infancy, the patient had recurrent respiratory infections and constipation. Immune evaluations at 2 months of age showed T cell lymphopenia and poor T cell function (
Table 1). In addition, T cell receptor excision circles, determined as previously described (
Roifman et al. 2012), were moderately reduced at 220 (normal > 400/0.5 μg DNA).
Pneumocystis jirovecii pneumonia prophylaxis was initiated. Subsequent laboratory evaluations demonstrated appropriate immunoglobulin levels as well as adequate production of antibodies to tetanus (4 IU/mL) and pneumococci (total IgG >270 mg/L) following vaccination. In addition, the patient had intermittent neutropenia of undetermined etiology that eventually resolved. By 3 years of age the patient’s number of CD4
+ T cells improved and T cell responses stabilized at 25% ± 5% of normal. The patient did not have an HLA-matched family donor and the parents elected not to pursue HSCT using alternative donors. At 7 years of age the patient had remained clinically well with no recurrent or severe infections or autoimmunity, with improved height (
Figure 2B). However, immune evaluations show persistent low number of CD8
+ T cells (
Table 1) with restricted repertoire (
Figure 1B), reduced CD45Ra naïve CD4
+ T cells (5.4% versus 43% in control) and CD8 T cells (9.6% versus 15.8%) as well as depressed T cell responses.
Appropriate standards for human experimentation were followed and informed consent was obtained prior to participating in the study.