Introduction
Chronic granulomatous disease (CGD) is a primary immunodeficiency caused by defects in one of the subunits of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex that results in the inability of phagocytes to generate superoxide, which is necessary for intracellular killing of both bacteria and fungi. These defects also predispose patients to granulomatous inflammation and autoimmunity (
Battersby et al. 2013;
Holland 2013;
Roos and de Boer 2013.
Mutations in each of the 5 structural genes of the NADPH oxidase have been found to cause CGD. X-linked inherited mutations in
gp91phox represent close to 65% of the cases, whereas autosomal recessive inheritance due to mutations in
p47phox account for 25% and the remainder are divided between mutations in
p67phox,
p22phox, and the sole case of
p40phox deficiency. There are no known autosomal dominant cases of CGD (
Holland 2013).
The X-linked form is generally considered to be clinically more severe; patients have earlier presentation, more severe infections, and earlier death than the autosomal recessive forms (
Holland 2013;
Roos and de Boer 2013). However, CGD is a heterogeneous condition with some patients presenting early in infancy whilst others do not present until late childhood or even early adulthood, most likely a reflection of the degree of residual NADPH oxidase function (
Battersby et al. 2013).
The most frequent sites of infection in CGD are the lung, skin, lymph nodes, and liver. The organisms causing the majority of these infections, at least in North America, are
Staphylococcus aureus,
Burkholderia cepacia complex,
Serratia marcescens,
Klebsiella species,
Nocardia species, and
Aspergillus species (
Holland 2013). However, many other catalase positive organisms have been described in these patients.
Of them all, the most common site of infection are the lungs, with
Aspergillus species being responsible for approximately 40% of the cases, although
Staphylococcus aureus and
Burkholderia cepacia are also important culprits (
Kang et al. 2011). Liver abscesses with granulomatous inflammation occur in approximately 35% of cases and most are caused by
Staphylococcus aureus. Up to 25% of patients suffer recurrence of these abscesses and they tend to develop multiple loculations, which makes percutaneous drainage difficult. The skin and lymph nodes are also involved in about 20% of patients, and the responsible infectious agents are usually
Staphylococcus aureus, Klebsiella species,
Serratia marcescens, Burkholderia cepacia, and fungi (
Kang et al. 2011;
Roos and de Boer 2013). Osteomyelitis is occasionally seen occurring either from hematogenous spread or contiguous invasion of bone.
Dysregulated granulomatous inflammation in CGD patients can be found in multiple tissues including the brain, lungs, liver, spleen, genitourinary tract, skin, and lymph nodes. Granulomas in CGD are typically noncaseating or nonnecrotizing and composed of multinucleated giant cells, and in most instances no pathogen is identified. Other complications associated with hyperinflammation experienced by some patients are conditions such as inflammatory bowel disease (IBD), lung fibrosis, and varied autoimmune conditions (
Kang et al. 2011;
Rieber et al. 2012;
Holland 2013).
Granulomatous lesions in the lungs, liver, and spleen have been observed to wax and wane even without intervention. Moreover, clinical symptoms frequently do not alert to the true extent of tissue damage. It is therefore recommended to order imaging early in the disease (
Towbin and Chaves 2010;
Kang et al. 2011).
Diagnosis of CGD is initially made by measuring the NADPH oxidase activity in phagocytes either by the direct measurement of superoxide production, ferricytochrome
c reduction, chemiluminescence, and nitroblue tetrazolium (NBT) reduction, or by dihydrorhodamine oxidation (DHR). Once the deficient NADPH oxidase activity is identified, confirmatory diagnosis is made by mutation analysis of the gene encoding for the subunits of this complex. The gene defect could be a predictor of the prognosis and, therefore, a useful tool in genetic counselling and prenatal diagnosis (
Roos and de Boer 2013).
Prophylactic treatment with trimethoprim–sulfamethoxazole and an azole antifungal agent such as itraconazole is the most effective modality of treatment to reduce the frequency of potentially fatal bacterial and fungal infections. Nevertheless, despite appropriate prophylaxis, many patients still experience at least one severe bacterial or fungal infection every 3–4 years (
Holland 2013;
Roos and de Boer 2013).
Currently, the only cure for CGD is an allogeneic hematopoietic stem cell transplantation (HSCT) (
Kang et al. 2011), and it is recommended to undergo this procedure as early as possible and prior to extension of end organ damage.
Gene therapy for CGD has not yet been proven to be a viable alternative to HSCT (
Grez et al. 2011).
Discussion
As evidenced by our patients, invasive bacterial and fungal infections are a significant threat for CGD patients. Radiological findings as well as the identification of the causative microorganisms, which often requires invasive procedures, are important diagnostic measures to guide optimal treatment (
Towbin and Chaves 2010;
Henriet et al. 2013).
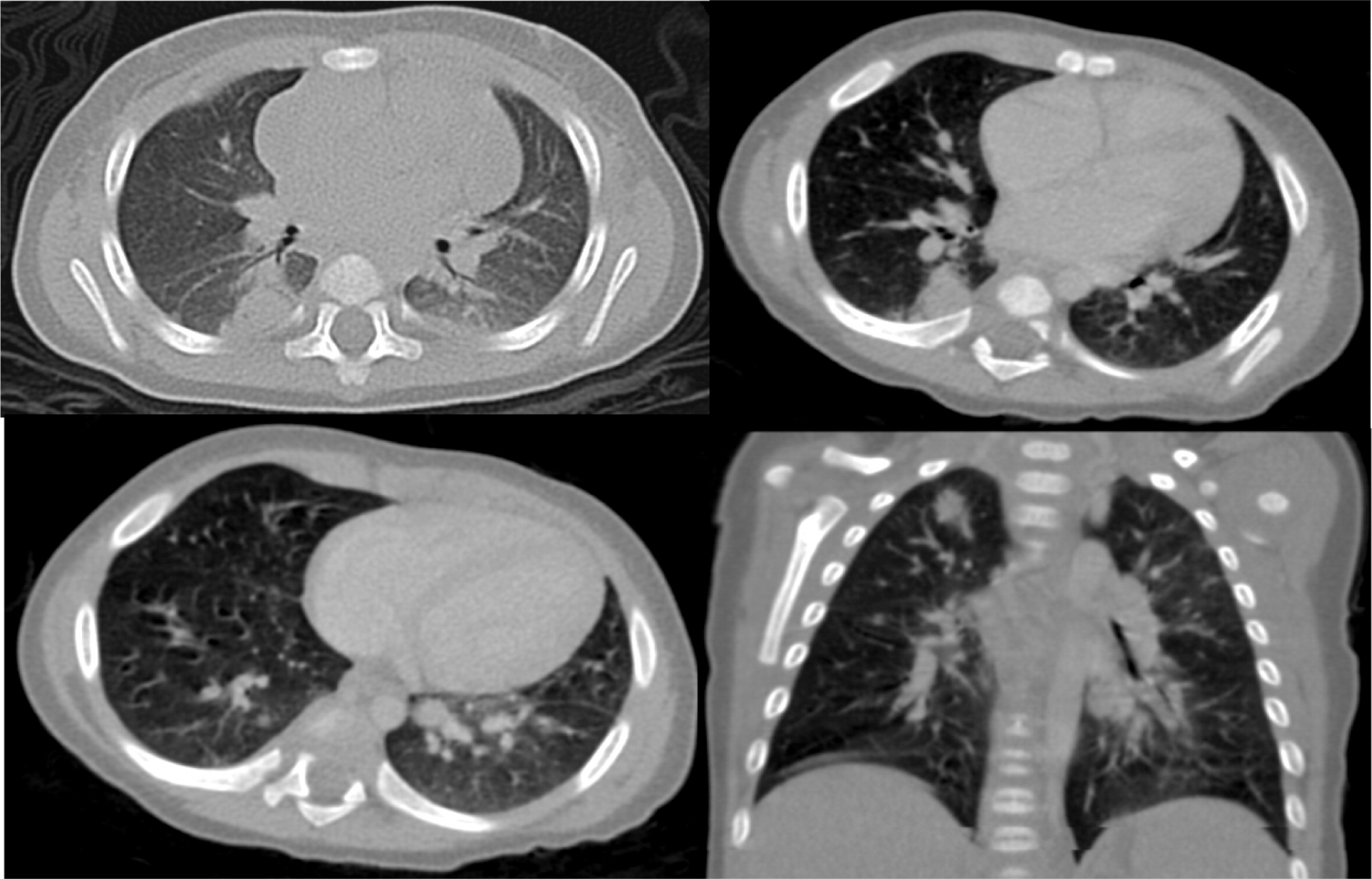
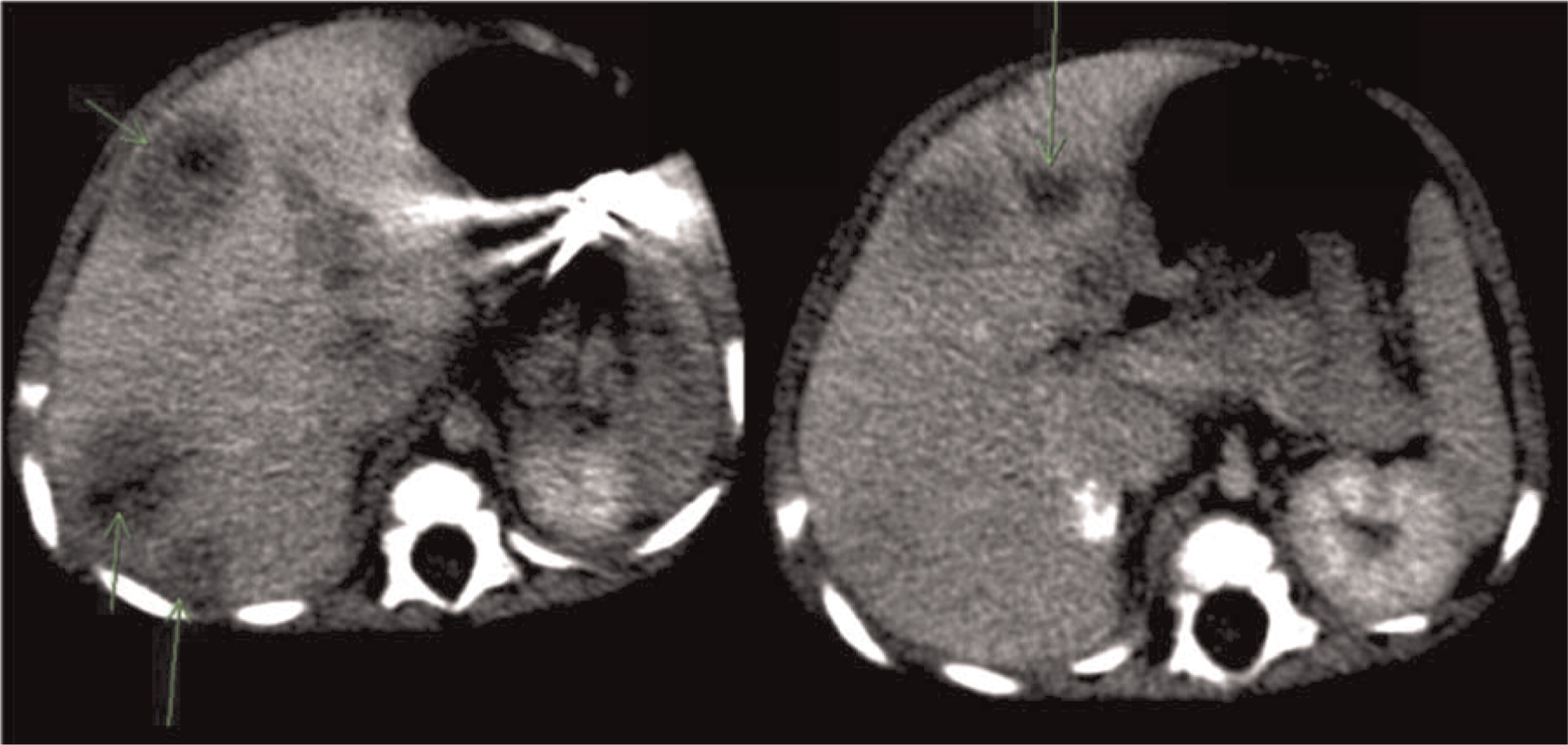
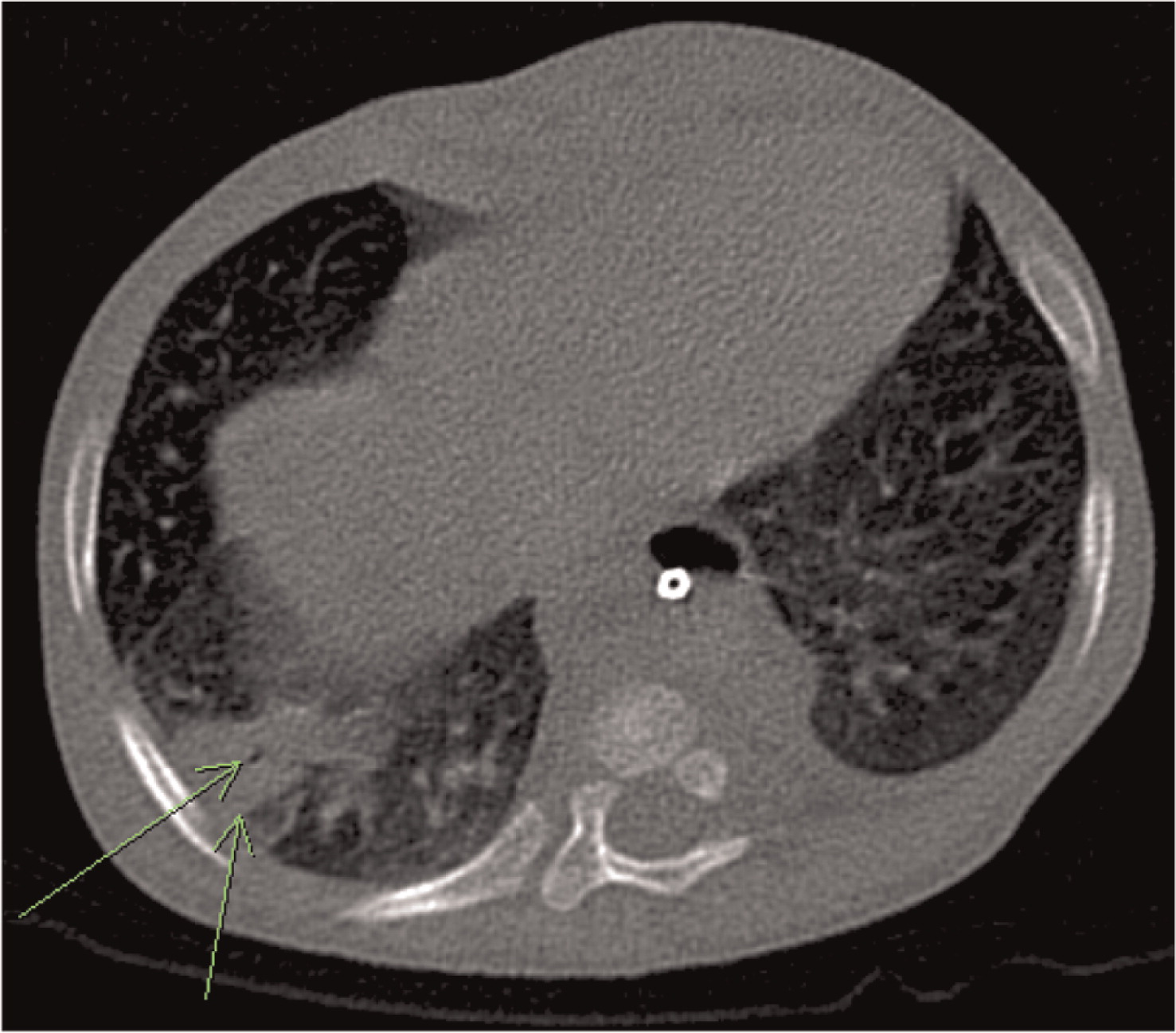
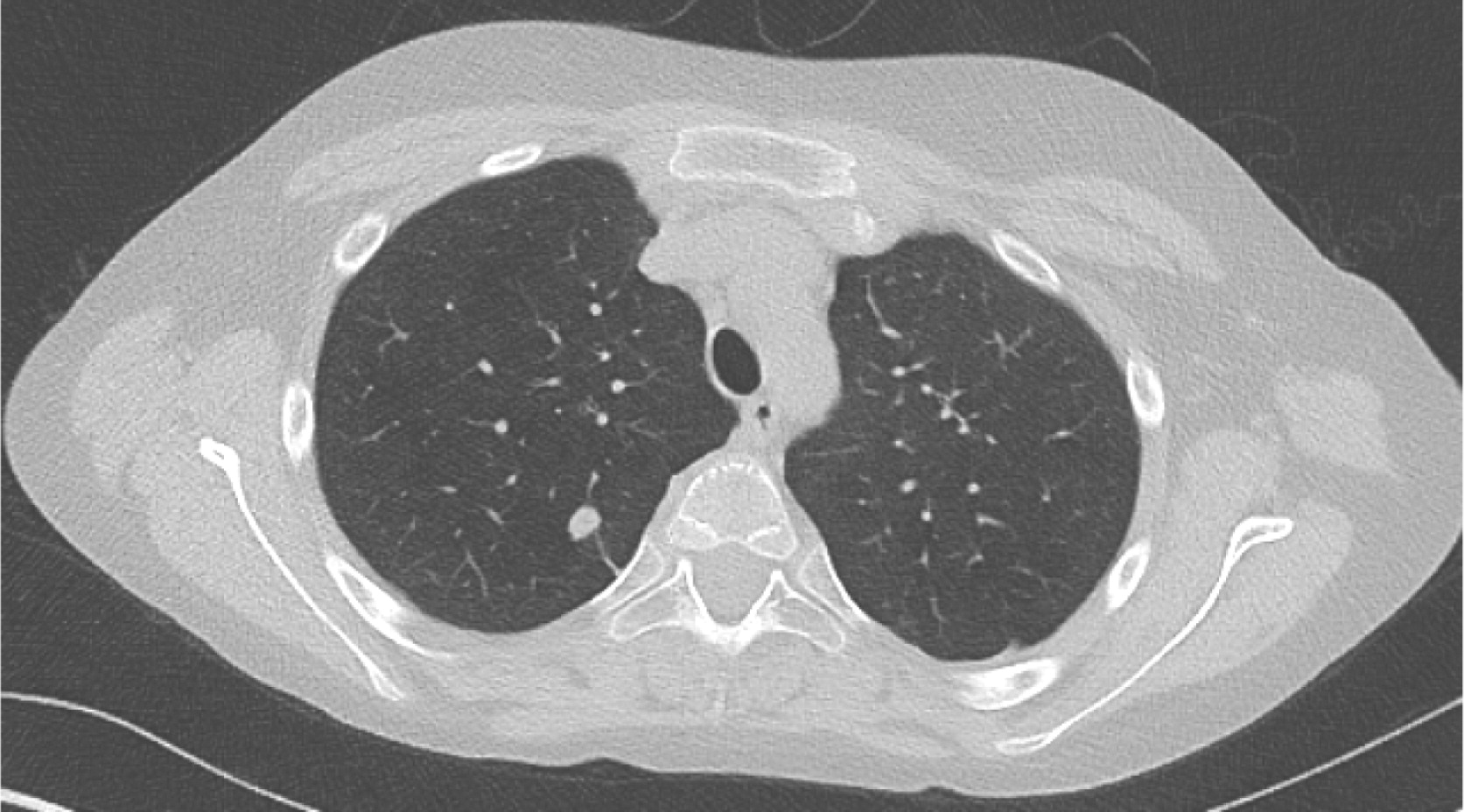
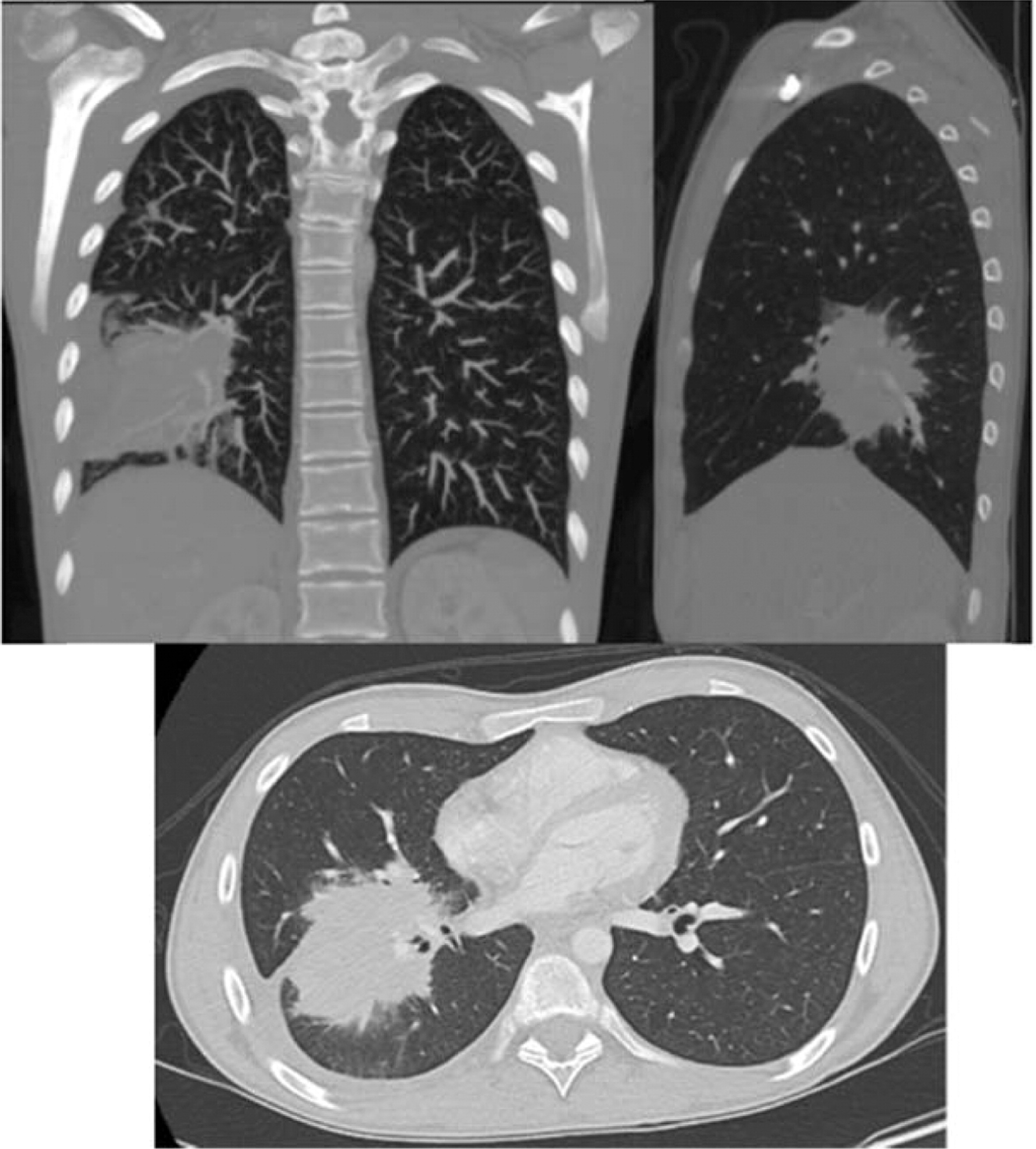
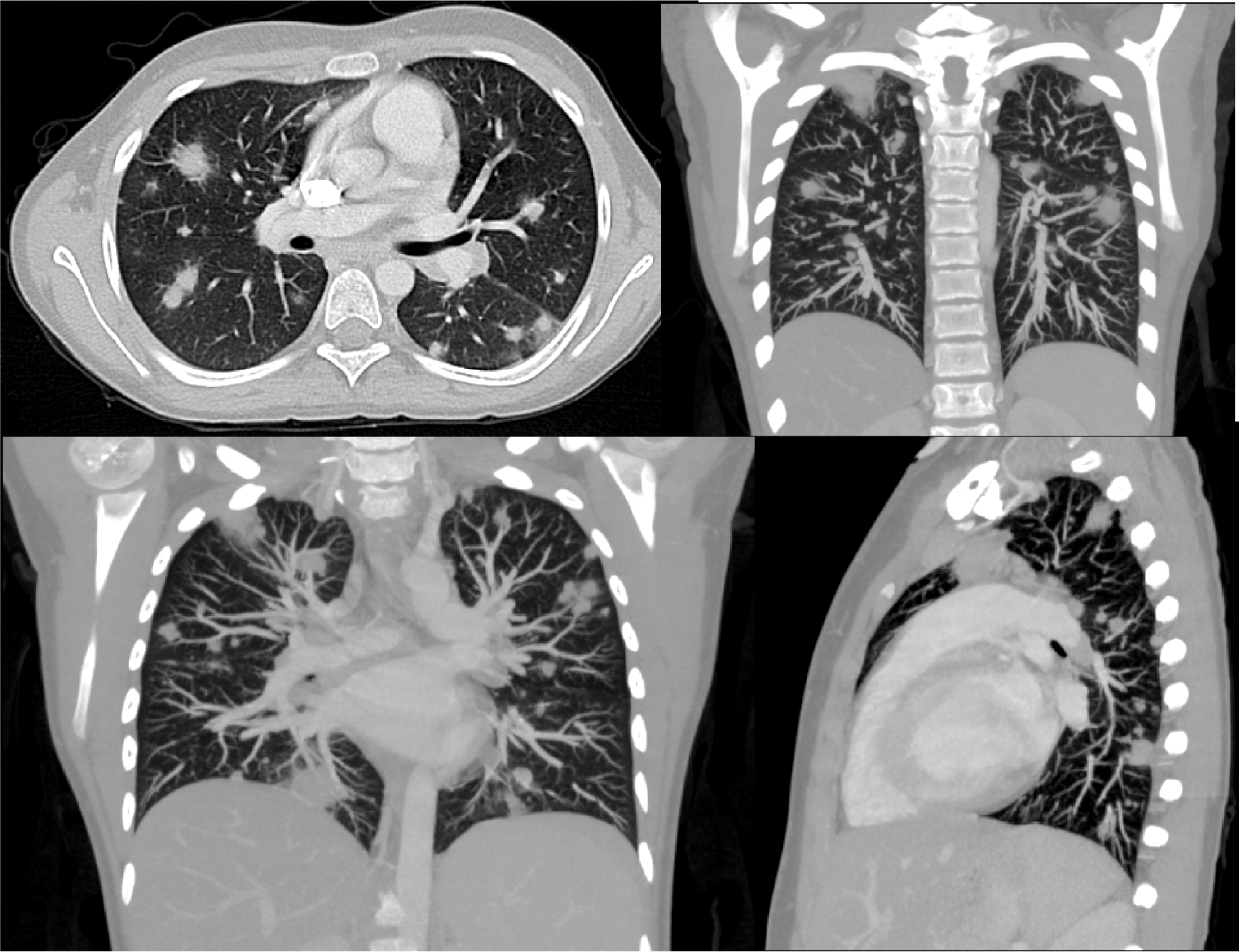
Imaging of the lungs is very important as pulmonary manifestations are the most common features in CGD, presenting in as many as 80% of patients and representing the most important cause of death (
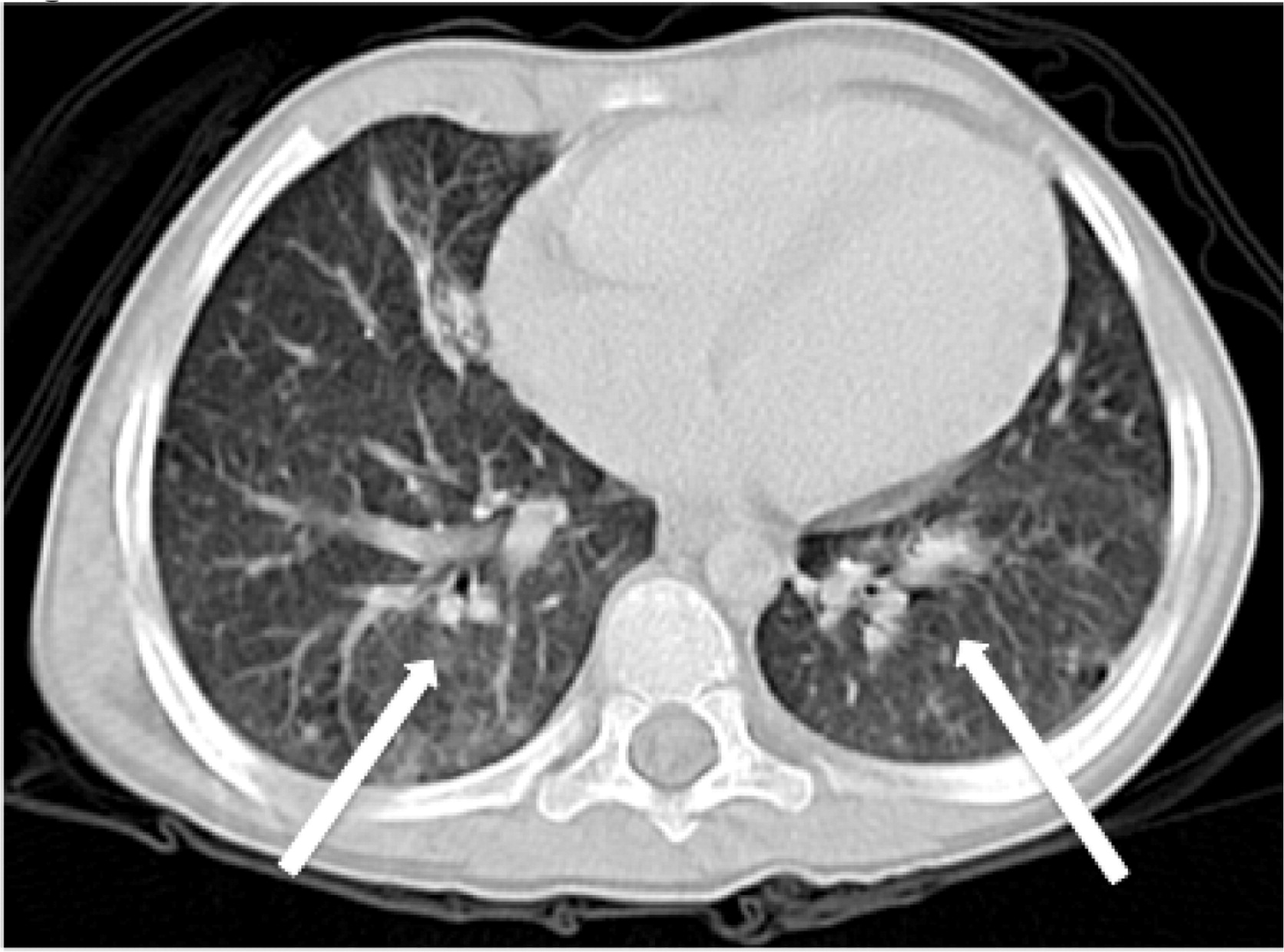
Winkelstein et al. 2000). Chest radiographs usually show consolidations, reticulonodular opacities, and scarring. CT findings include consolidation, ground-glass opacities, tree-in-bud pattern, centrilobular or random nodules, bronchiectasis, septal thickening, air trapping, or scarring. Complications such as abscess formation, empyema, or lung fibrosis may also be found and are quite unique to CGD where contiguous spread to the chest wall and associated osteomyelitis of ribs and vertebrae may occur (
Towbin and Chaves 2010;
Mahdaviani et al. 2013).
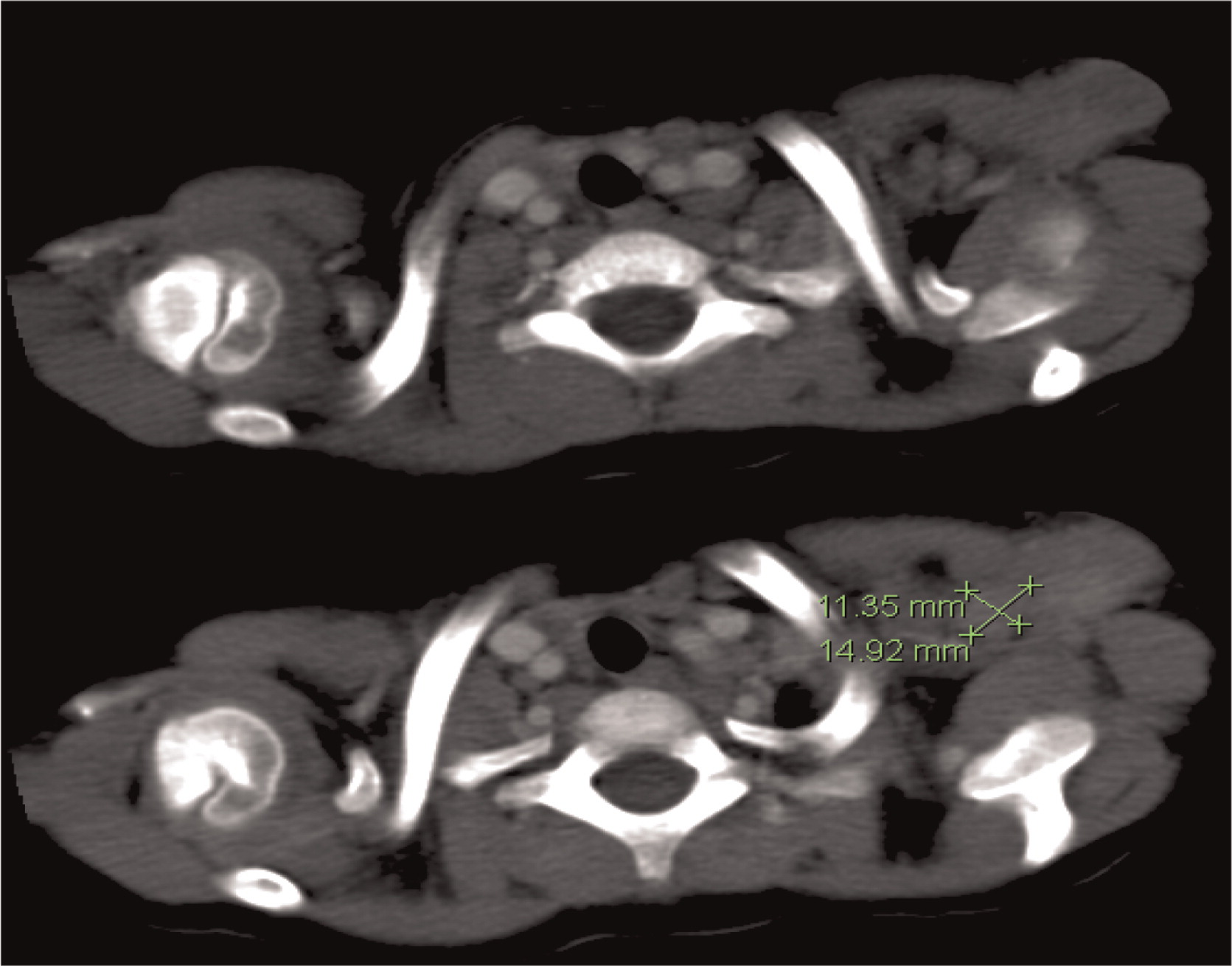
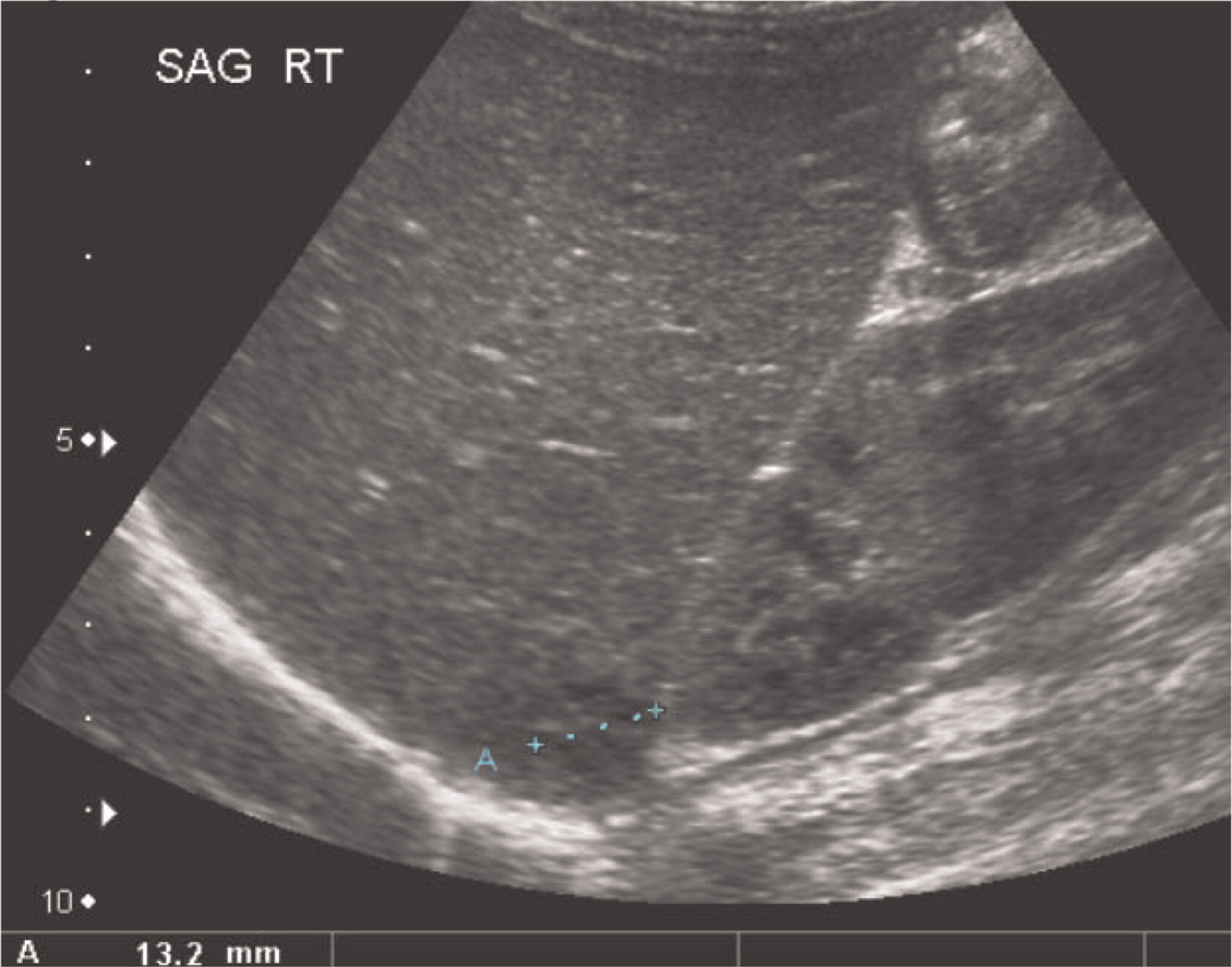
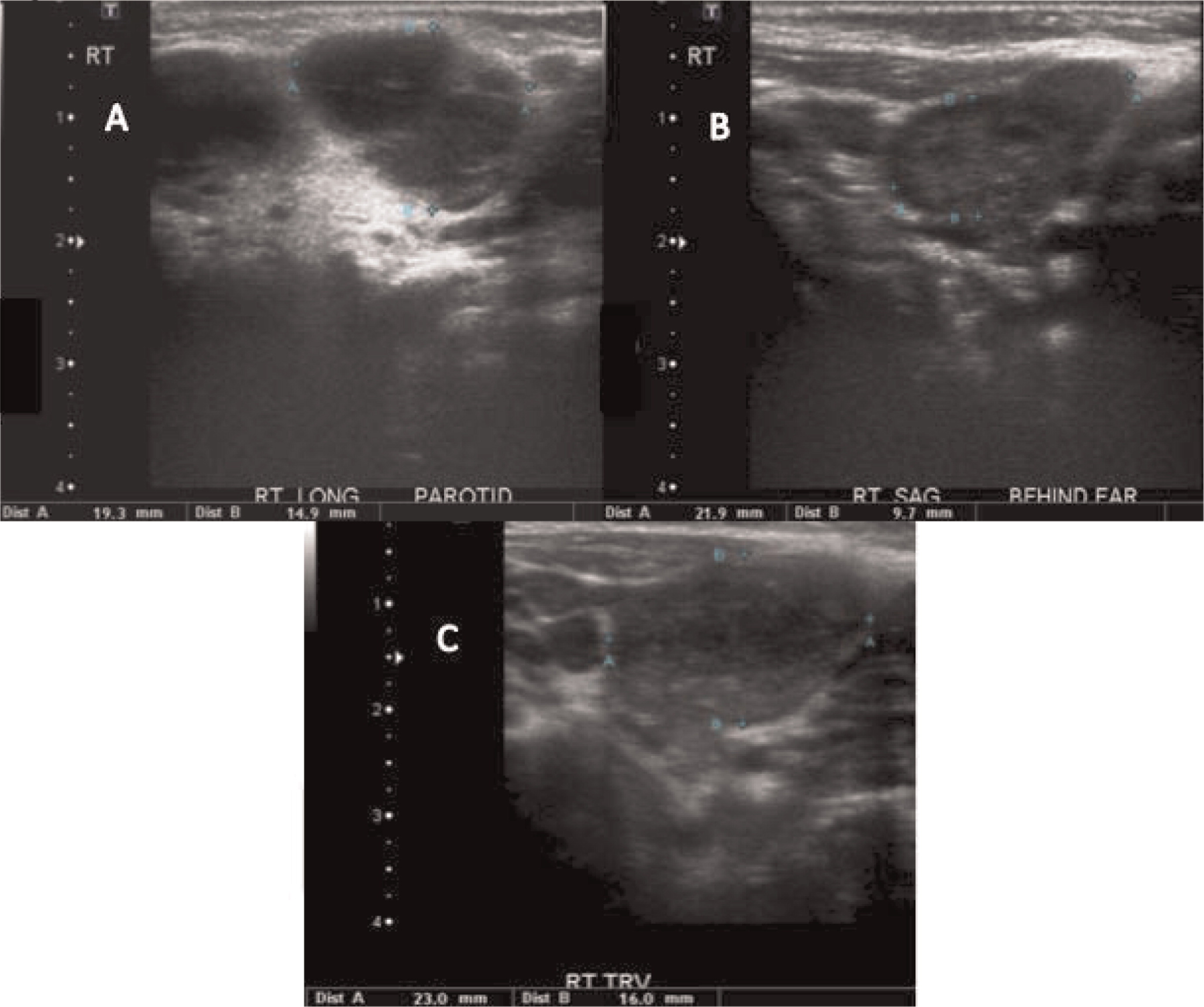
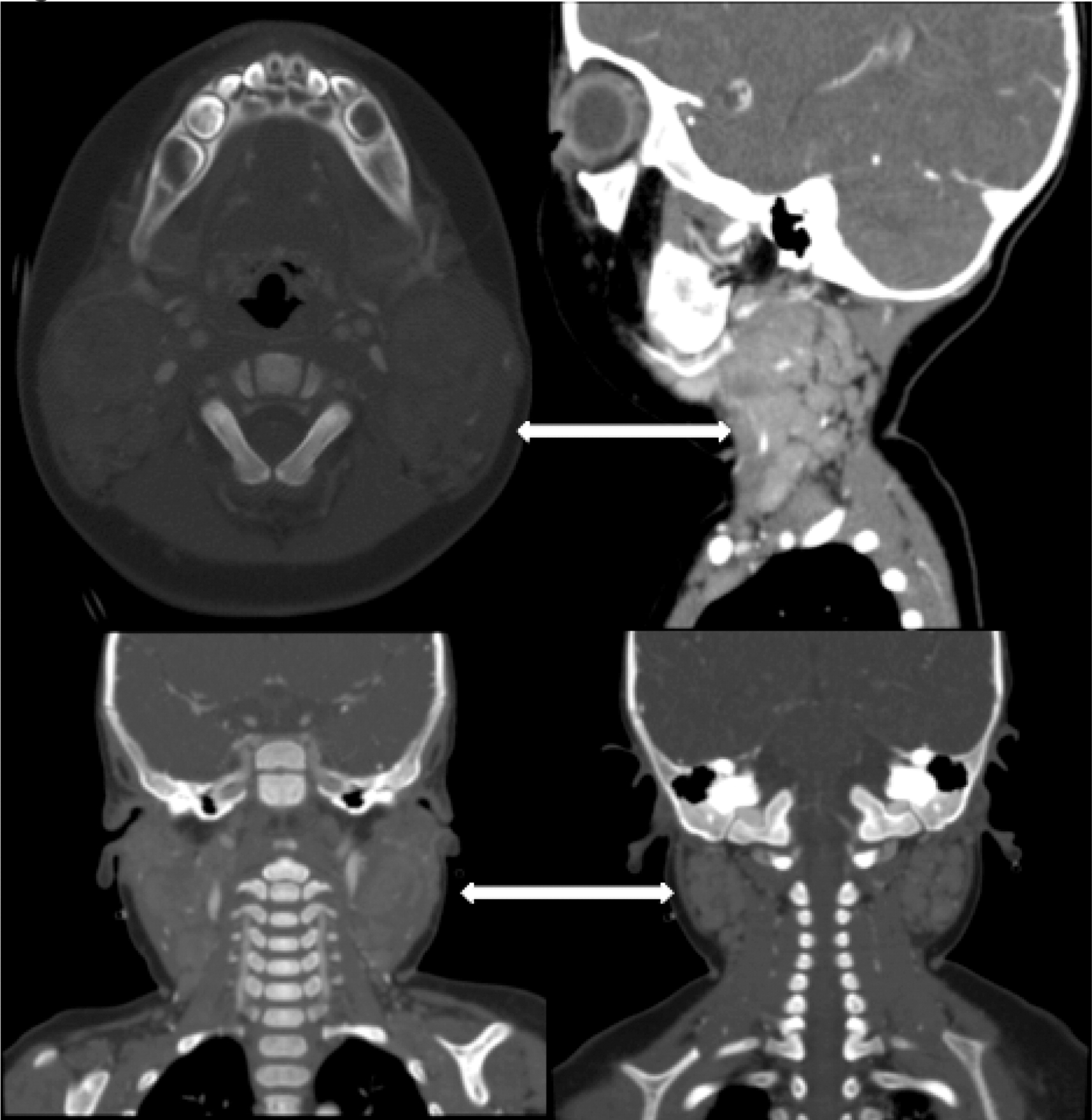
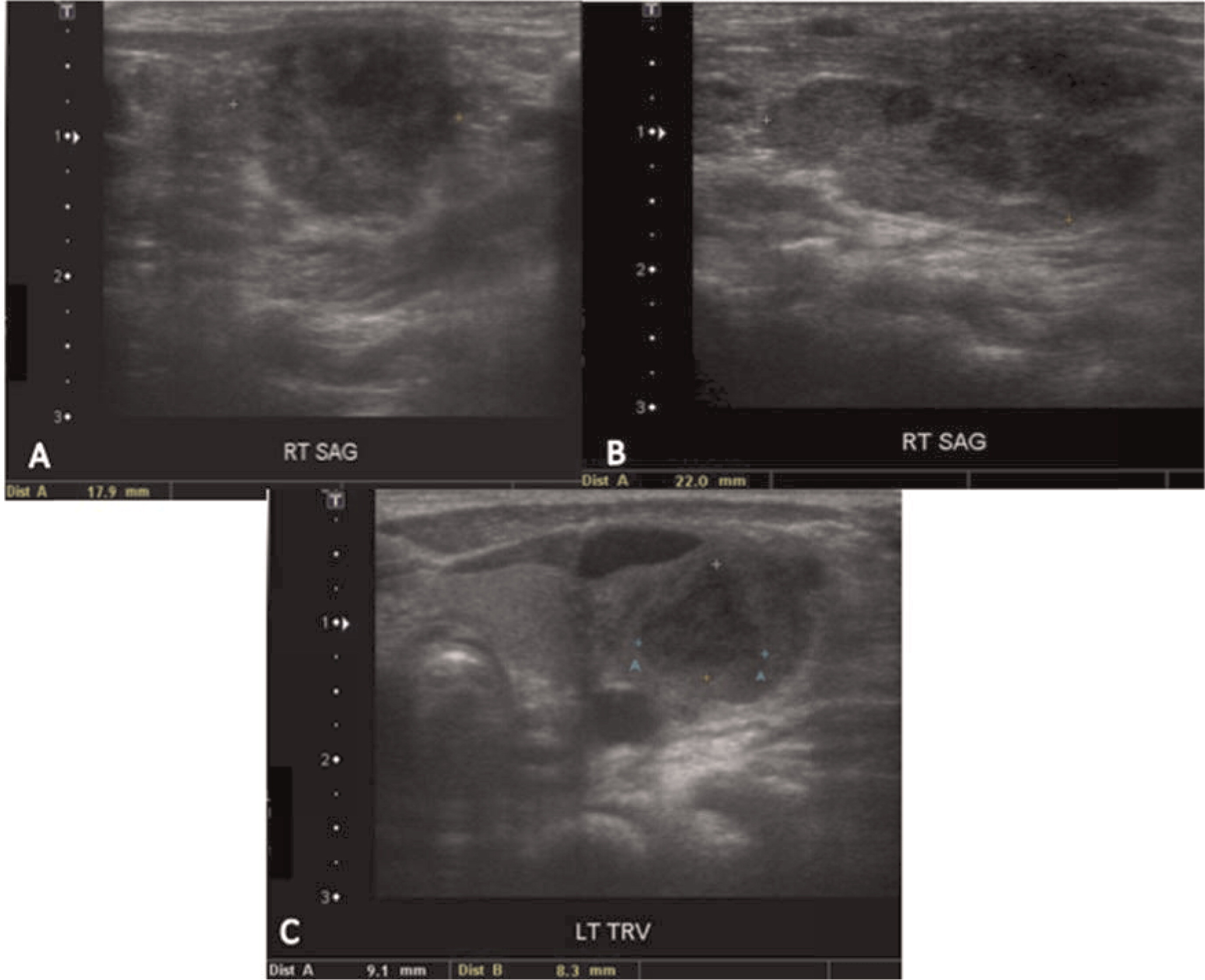
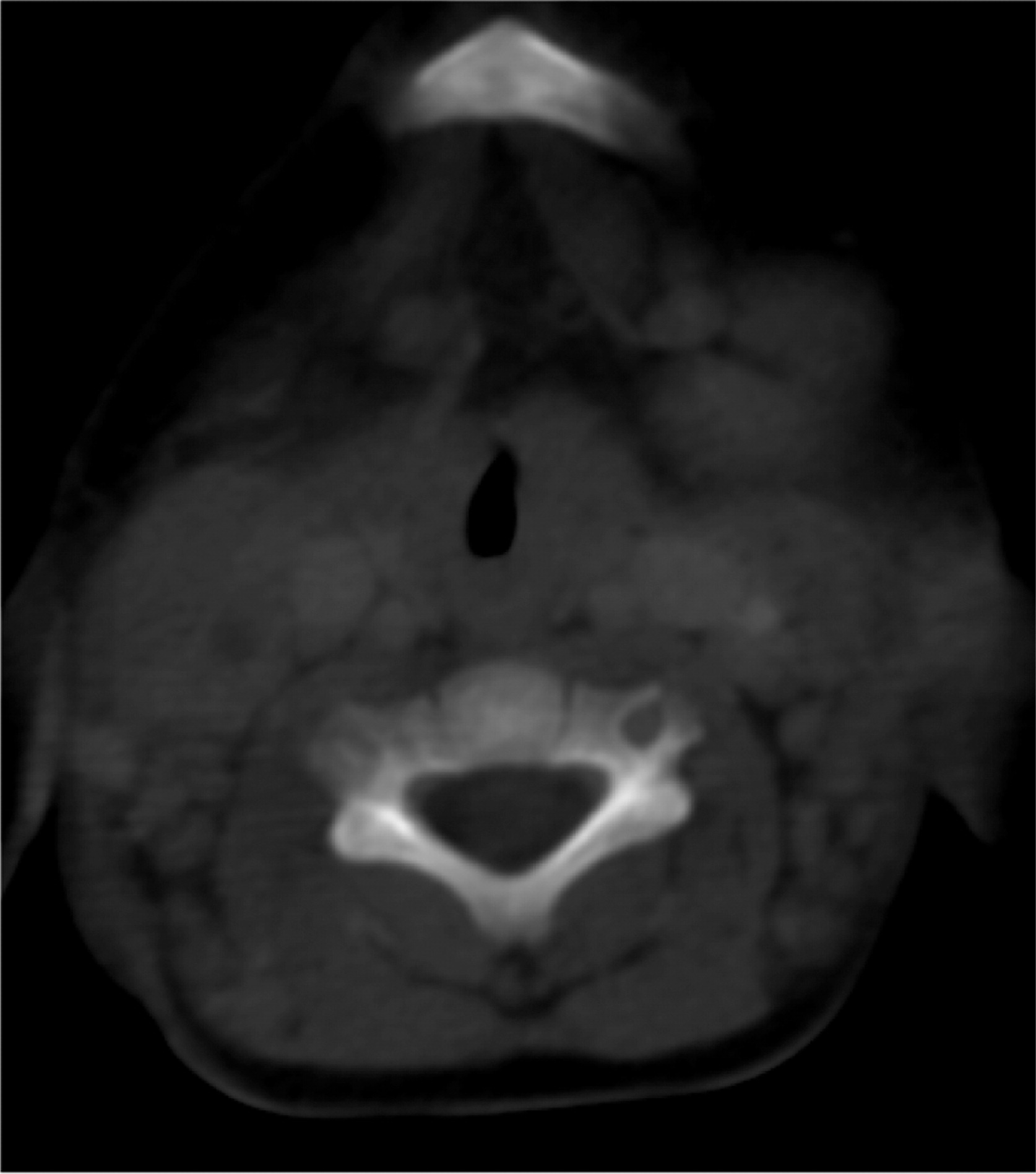
More than half of the patients with CGD might show changes in the lymph nodes as evidenced by imaging. Most of these changes are due to suppurative adenitis. The cervical nodal chain is most frequently involved, although other nodal sites can be affected. On US, internal debris are usually present with compression of the infected lymph node. Unique features to CGD are the findings by CT of thick, enhanced septae and calcifications in more chronic disease. Nonsuppurative infection with granuloma formation can also occur in lymph nodes (
Winkelstein et al. 2000;
Towbin and Chaves 2010).
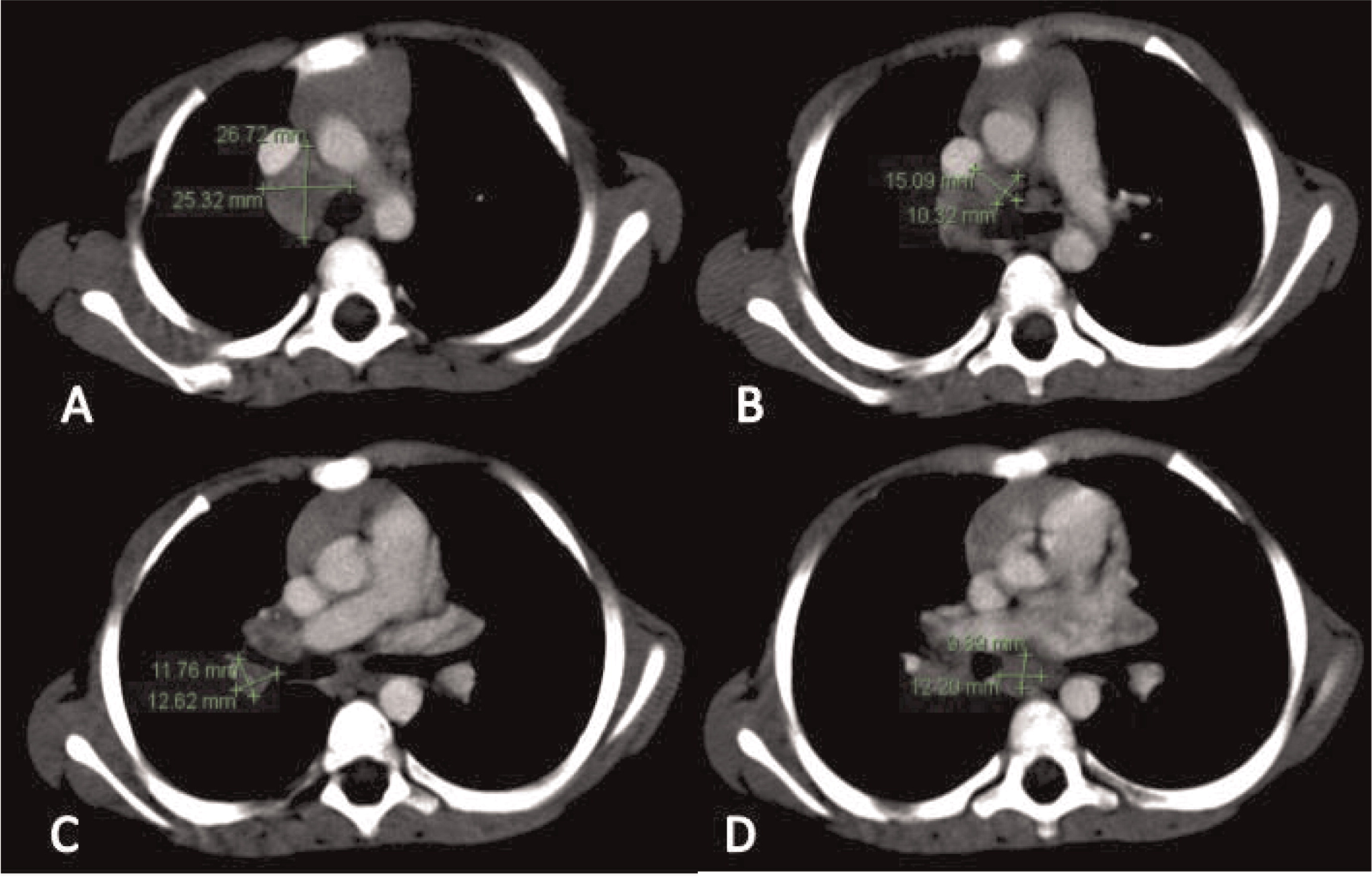
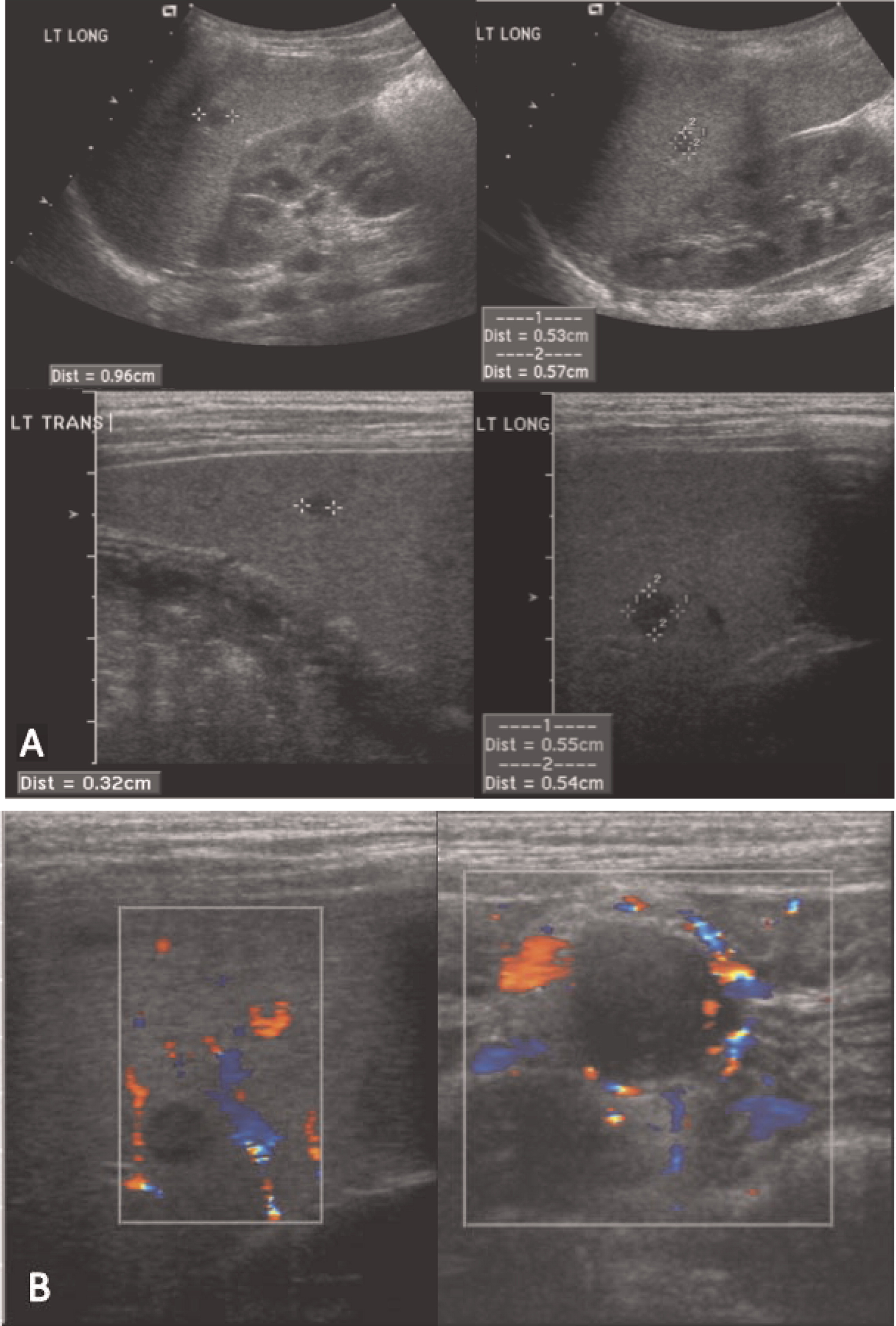
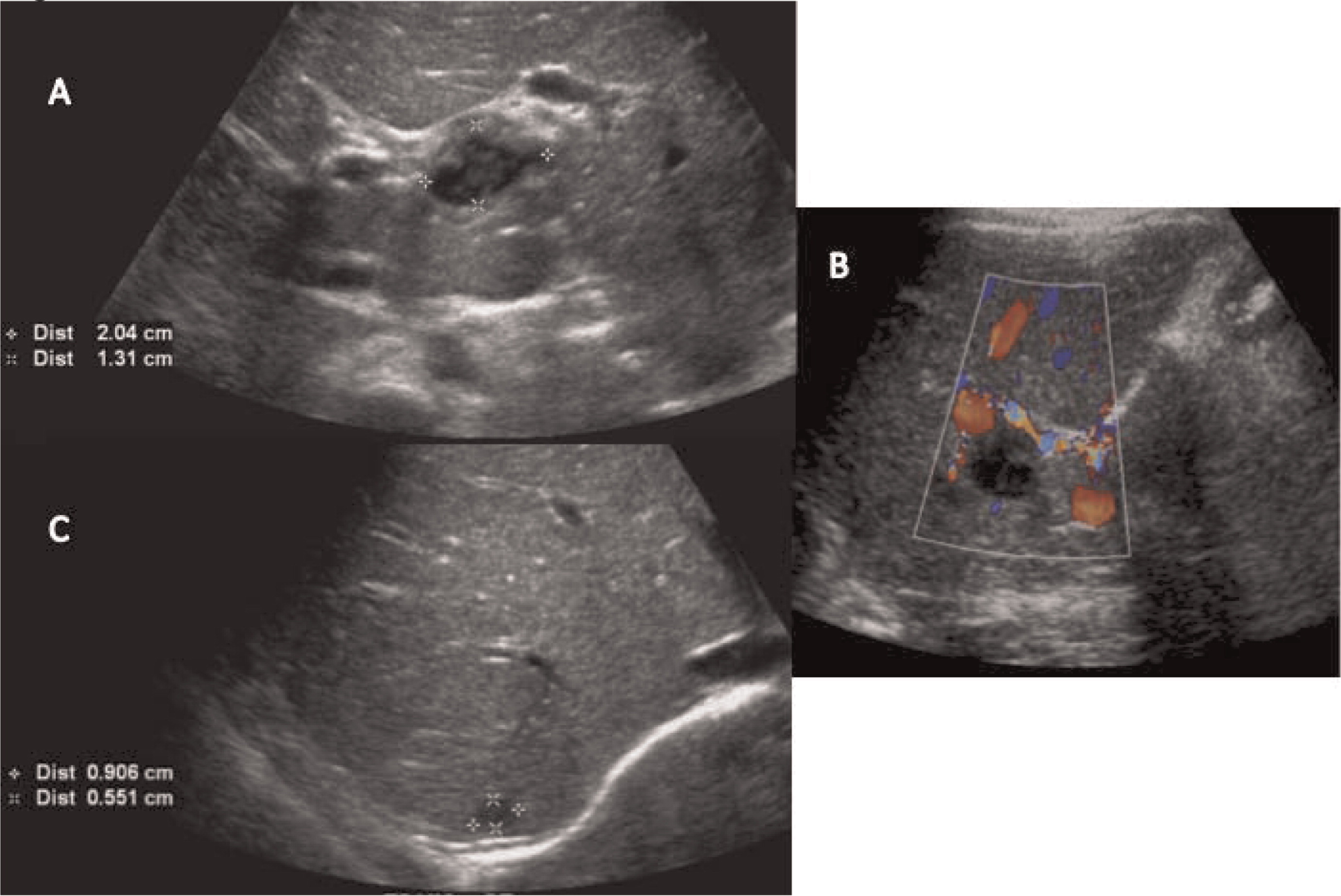
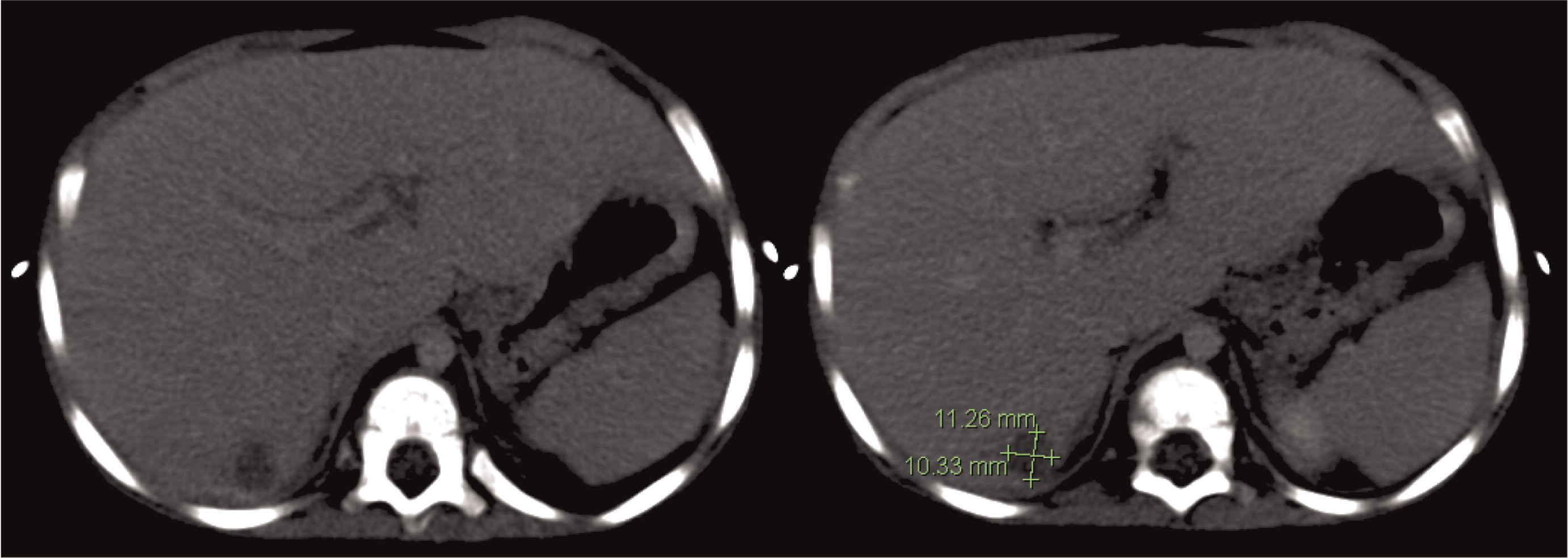
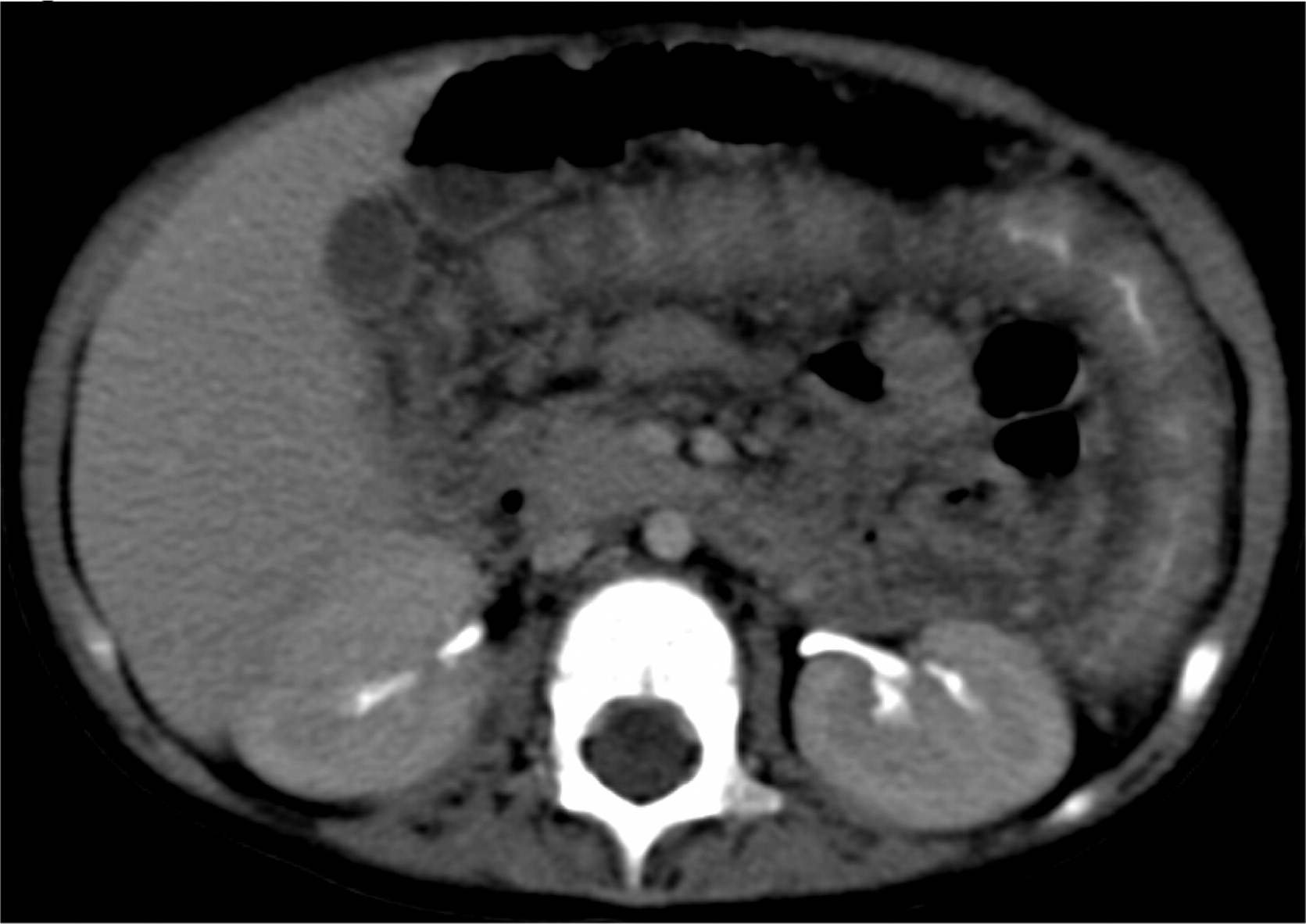
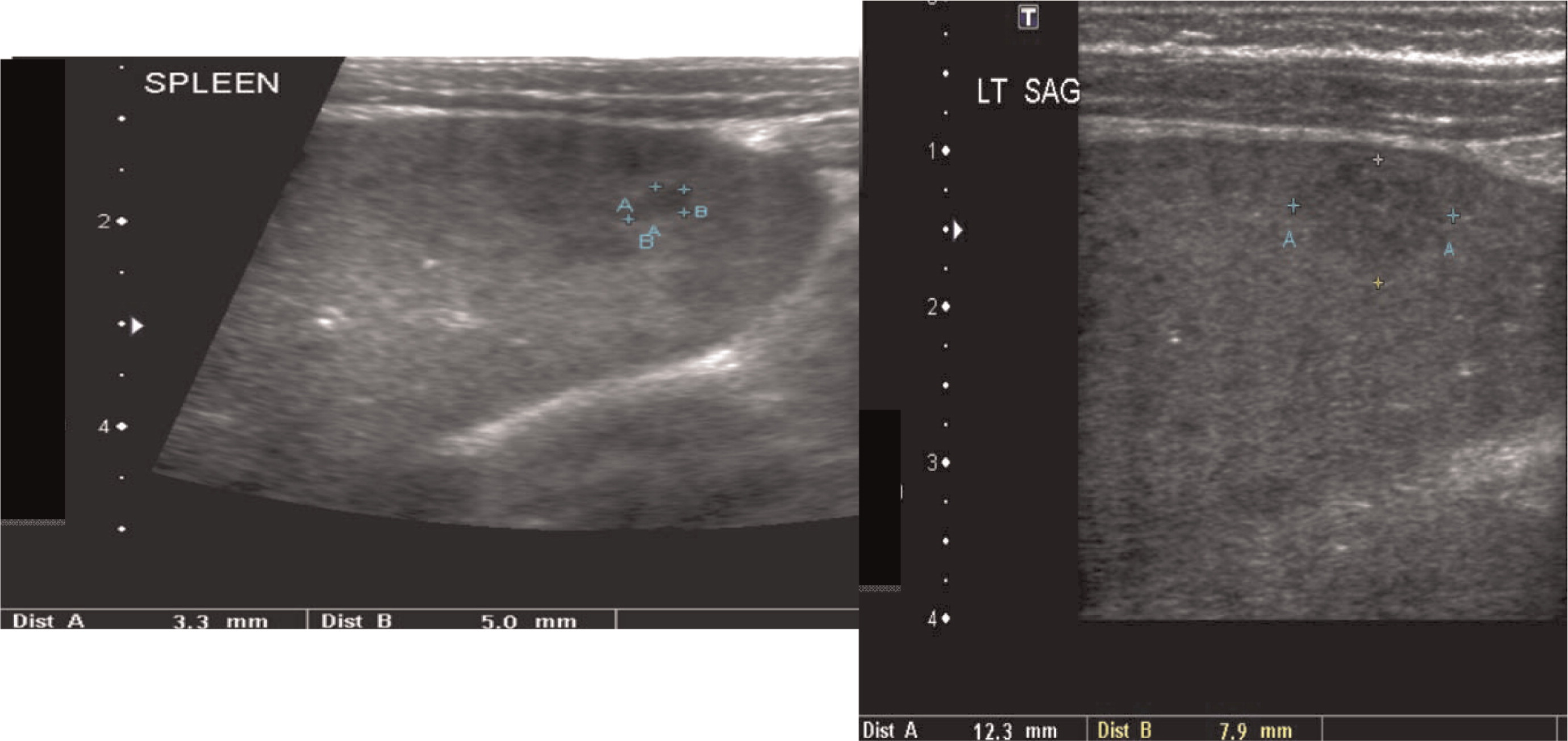
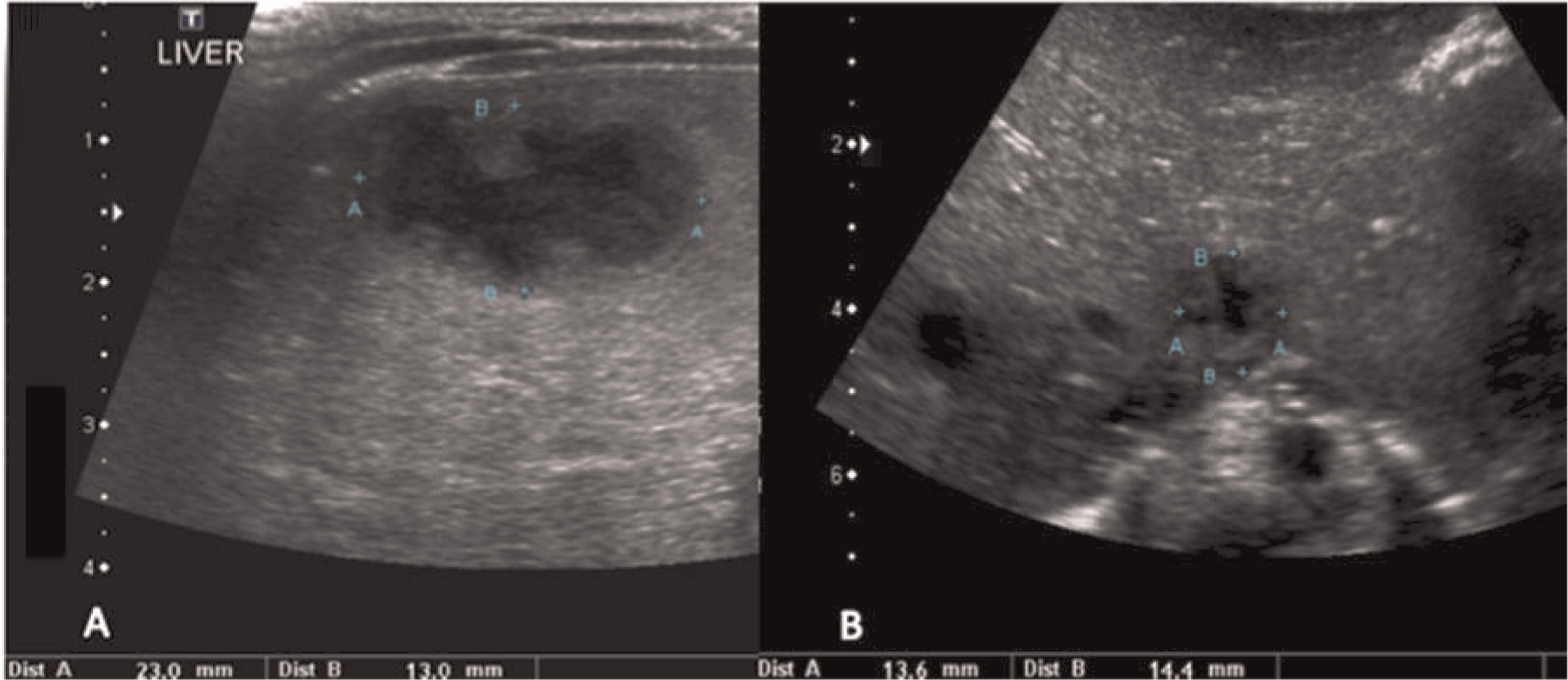
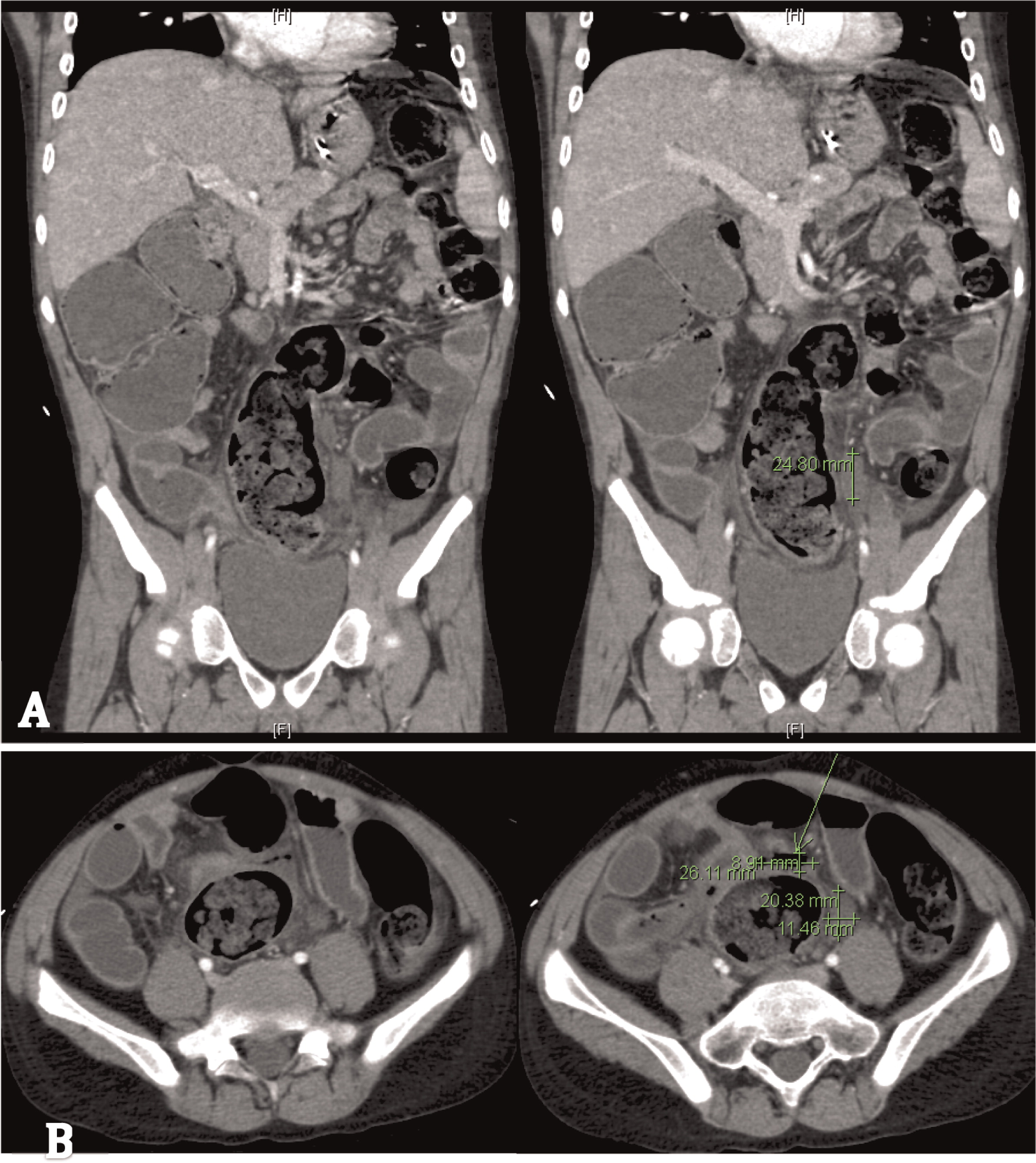
Multiple abscesses and (or) granulomas may be present in the liver of 25%–50% patients (
Winkelstein et al. 2000;
Towbin and Chaves 2010). Sporadic hepatic abscesses, without an inciting event such as trauma or systemic infection, are uncommon in children and thus, when found in a sporadic manner, they should raise the possibility of CGD. As shown in our patients, these lesions may also affect the spleen in up to one-third of patients. Interestingly, similar to the lungs, granulomas in the liver and spleen tend to wax and wane over time, and they generally disappear after treatment with HSCT.
As for the gastrointestinal tract, the most common imaging findings are bowel wall thickening mimicking IBD and thickening of the wall of the gastric antrum, the latter being a unique feature in CGD patients (
Winkelstein et al. 2000;
Towbin and Chaves 2010). Upper GI imaging studies can show gastric outlet obstruction with gastric dilatation, delayed gastric emptying, circumferential antral narrowing, and thickened gastric folds (
Towbin and Chaves 2010).
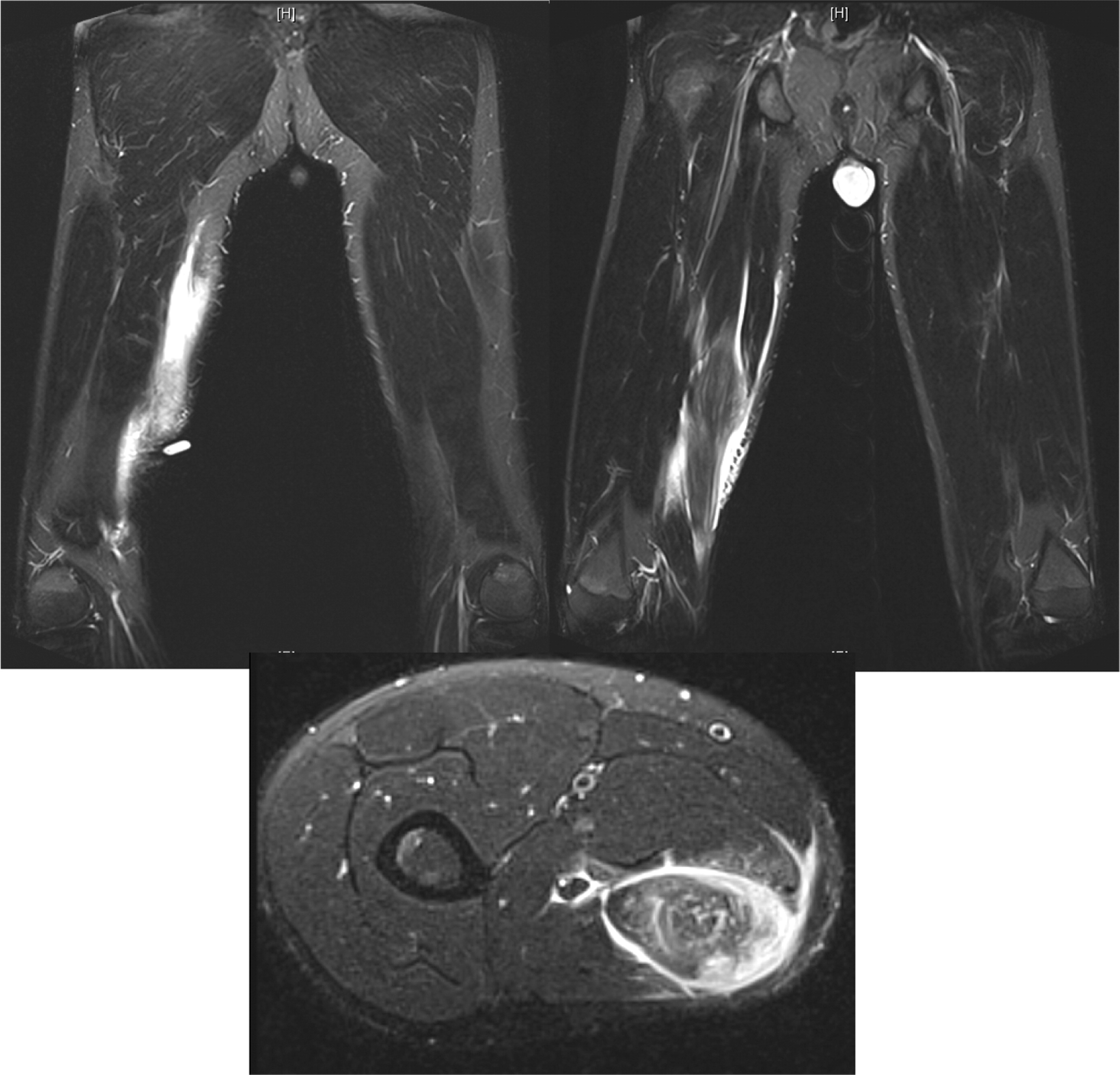
Osteomyelitis in CGD is usually caused by either
Serratia marcescens or
Aspergillus and localized to the lower extremities, chest wall, and vertebrae (
Winkelstein et al. 2000;
Towbin and Chaves 2010).To our knowledge, muscle lesions such as the one shown here in patient 6 have not been previously reported.
This work illustrates how imaging studies are critical tools for diagnosis as well as management of patients with CGD.