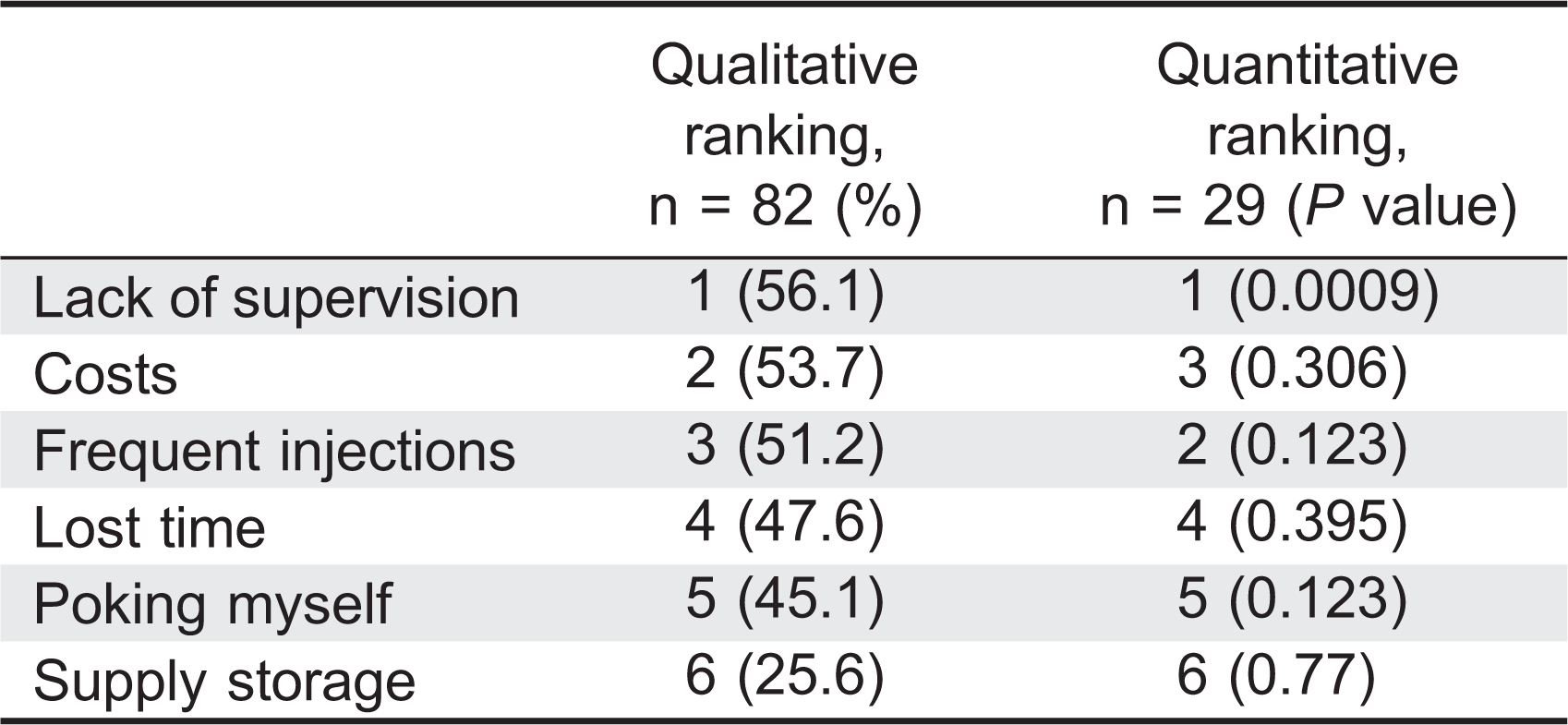
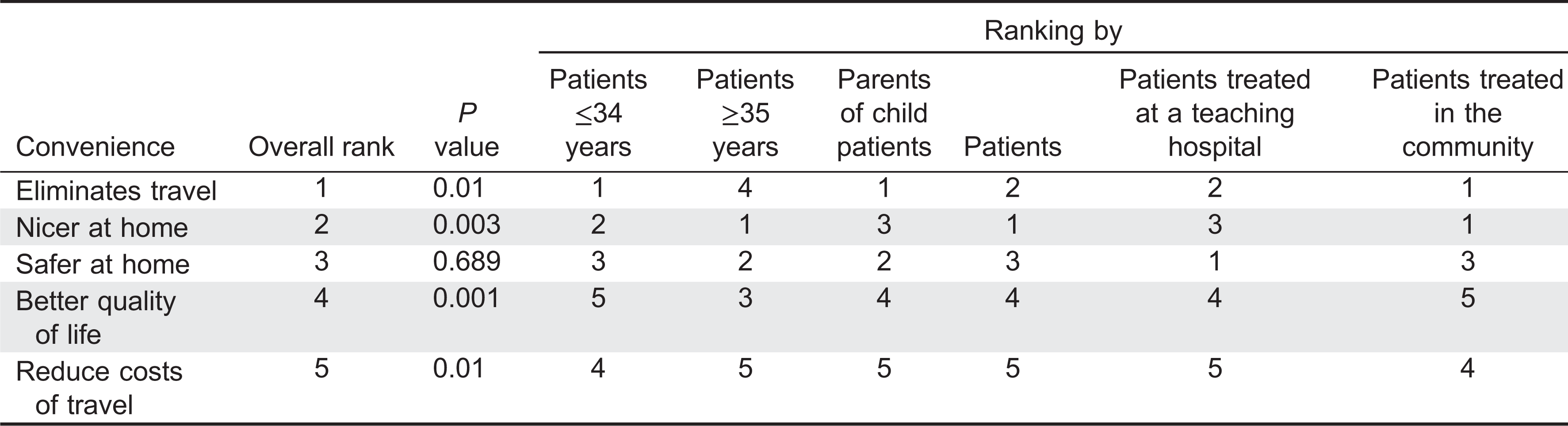
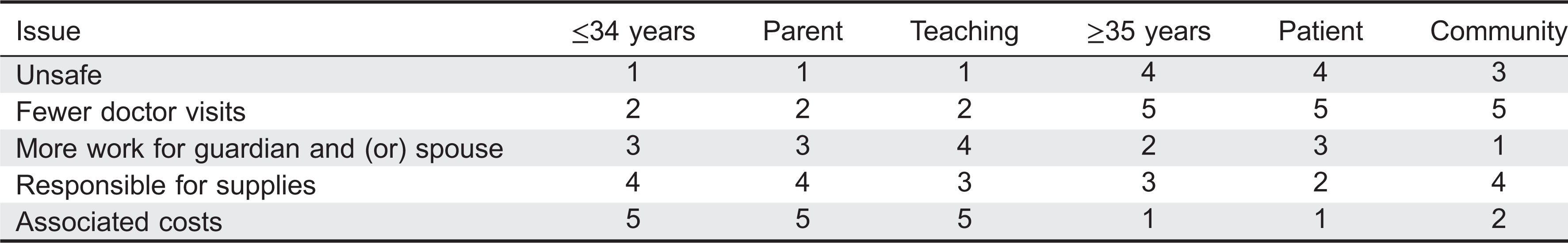
To account for patient dissatisfaction with current IVIG therapy, subjects were asked to select the most unpleasant experiences related to IVIG therapy. They were given 6 choices and requested to select all that apply. An option to rank their selections was provided with 1 being most unpleasant and 6 being the least unpleasant. Qualitative ranking was calculated for all answers and quantitative ranking was only calculated for the subjects who ranked their choices. Lost time and the infusions themselves were listed as major concerns (ranked first and second, respectively), whereas the hospital registration process was the least important issue (ranked sixth) (
Table 2). Travel time ranked fourth. Qualitative and quantitative analysis of this data could not consistently rank a placement for waiting time and adverse events (
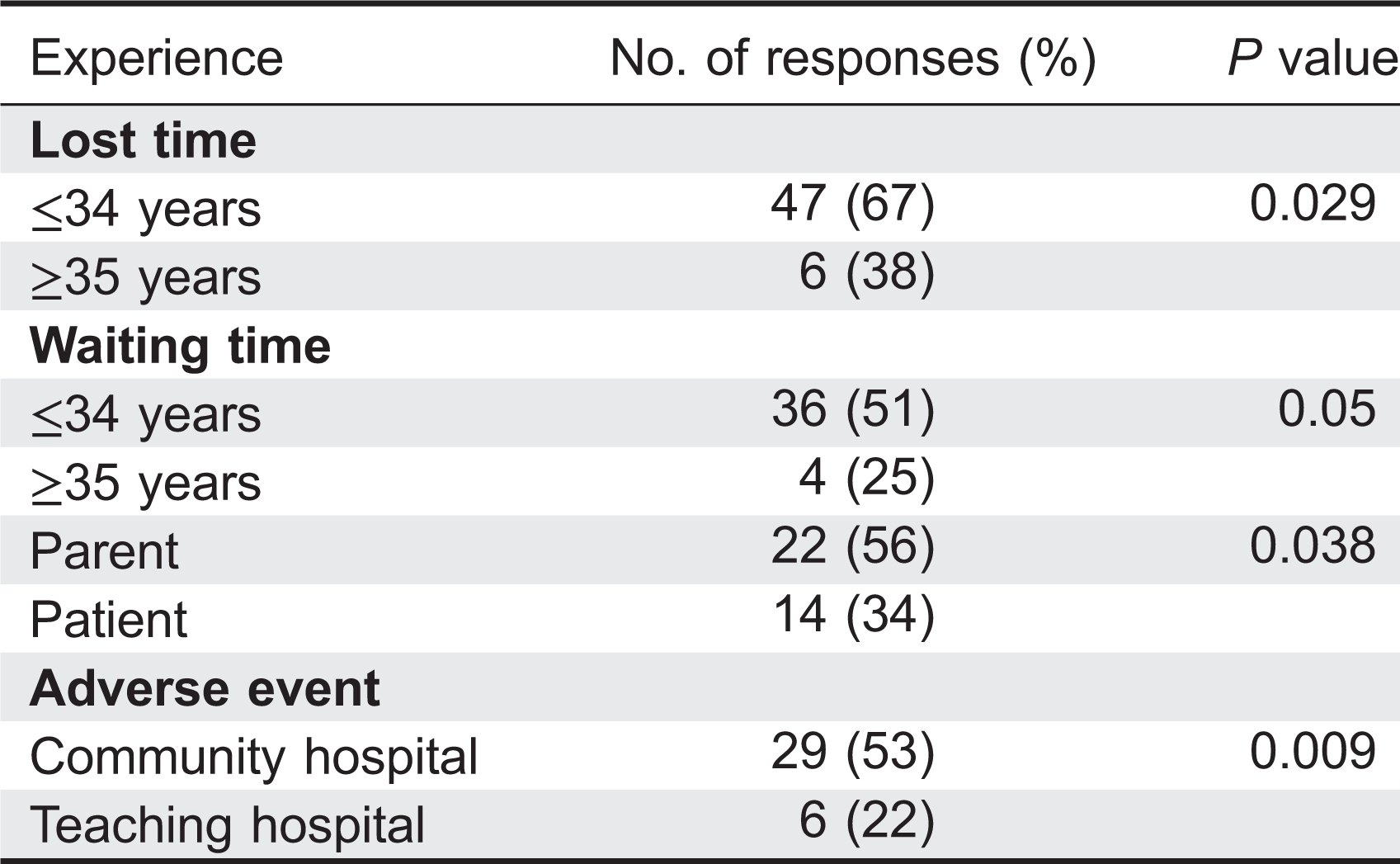
Table 2). Waiting time as a concern placed third in the qualitative analysis and fifth quantitatively out of six options. Adverse reactions showed reversed results ranking fifth for the qualitative analysis and third for the quantitative analysis. When analyzing data according to age, patients under 35 years were significantly more concerned with time (waiting time and lost time) than patients 35 years and older (
P = 0.05 and 0.029, respectively,
Table 3). Similarly, waiting time was significantly more important to parents rather than to patients (
P = 0.038). When analysis was performed according to location of treatment, 53% of patients receiving IVIG in the community were more concerned with ARs when compared with the 22% of referral center based patients (
P = 0.009).
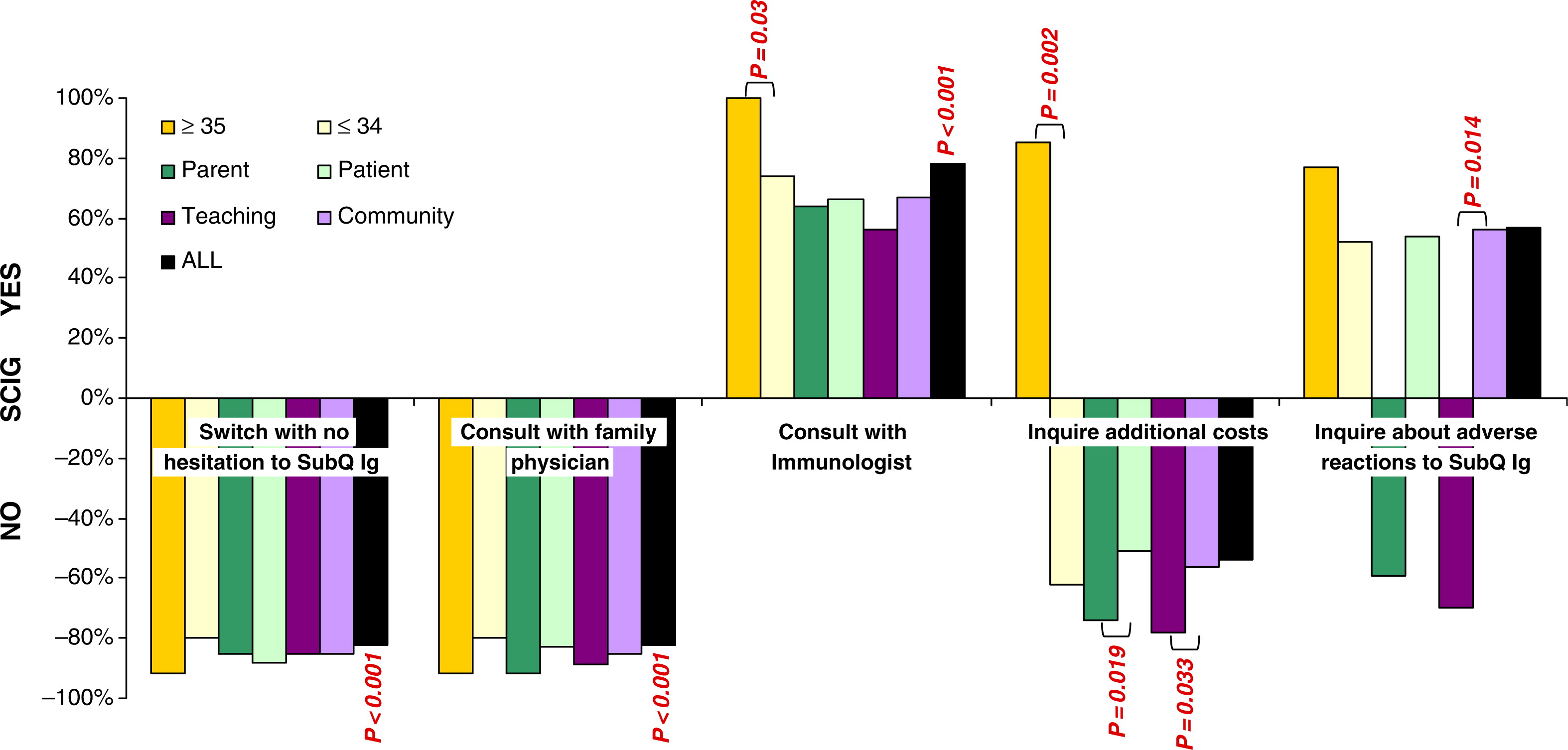
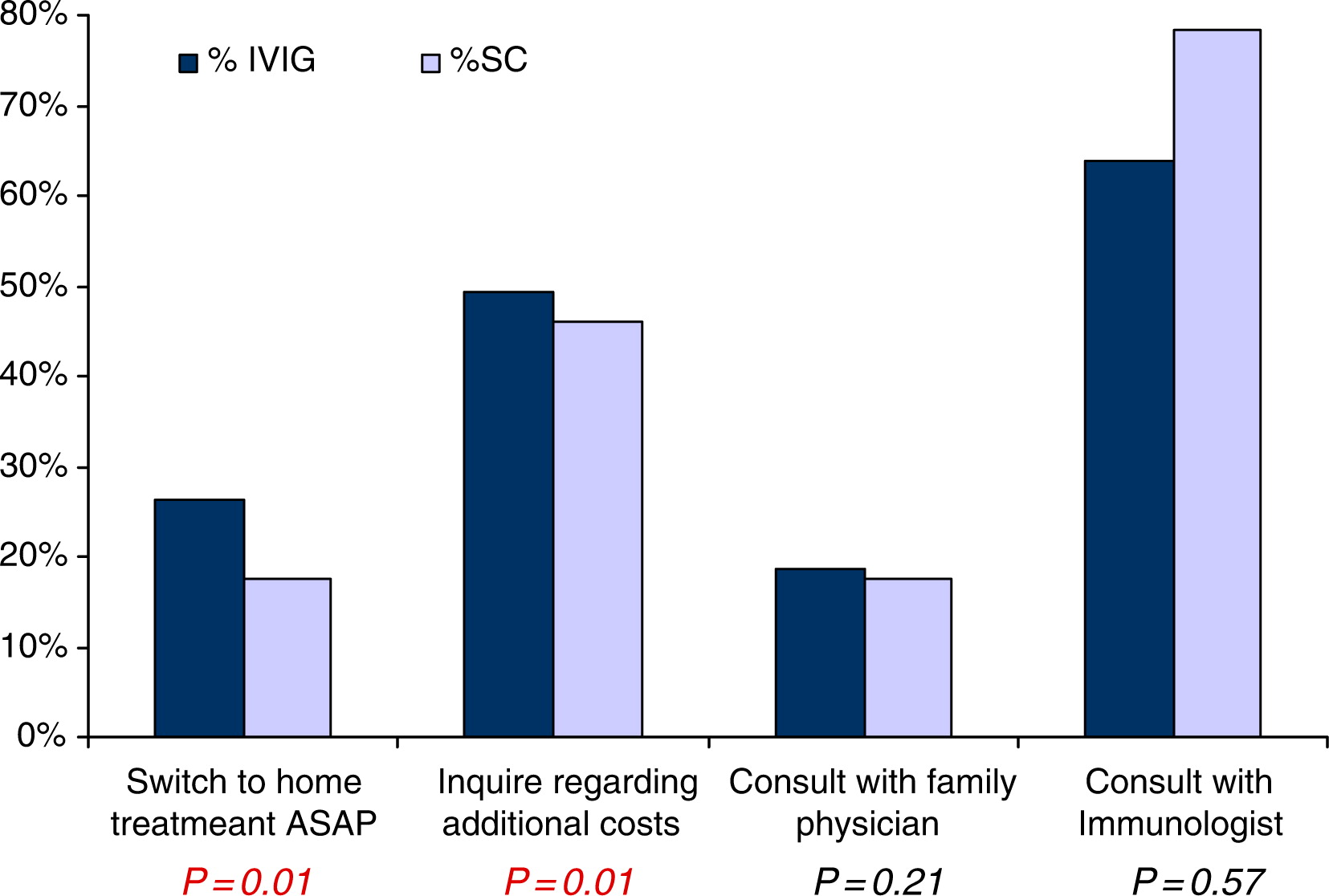
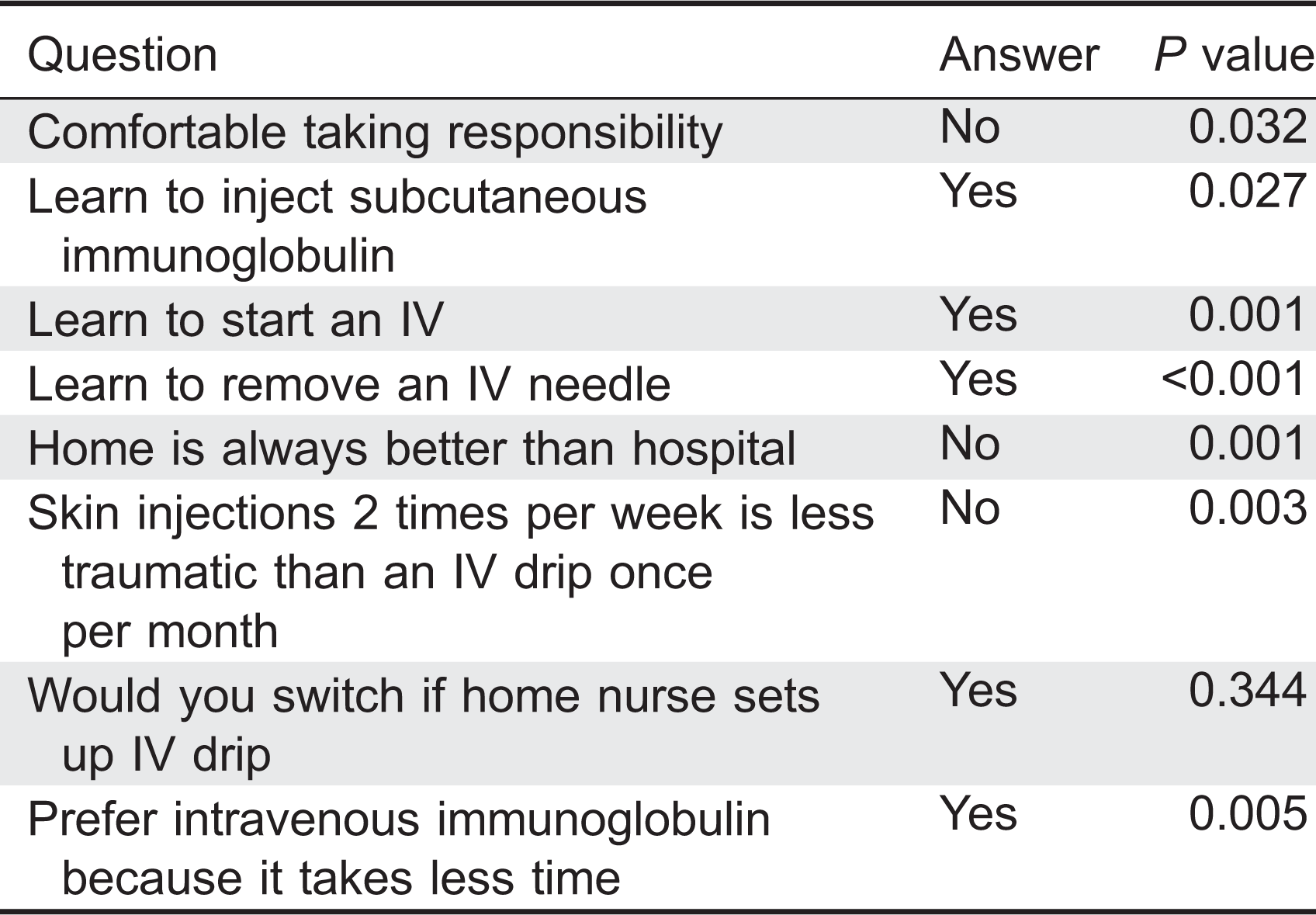
Patients were asked which strategy they would adopt if home IVIG became available. Only 64% of all patients agreed that they would switch from hospital- to home-based treatment, but only after consulting with their immunologist (
P = 0.006,
Figure 1). The majority of patients said they would not switch to home from hospital treatment based on a recommendation from their family physician (
P < 0.001), if a nurse was present (
P < 0.001), if a family member was trained to perform venipuncture (
P < 0.001), even after home therapy was proven successfully in Canada (
P = 0.0007), or they would switch right away without hesitation (
P < 0.001). Possible costs associated with home therapy were also not listed as a reason to change from hospital to home; however, this was not statistically significant (
Figure 1). By examining age subpopulations, the costs associated with home therapy stand out as one of the major concerns for patients 35 years and older and those receiving therapy in the community (
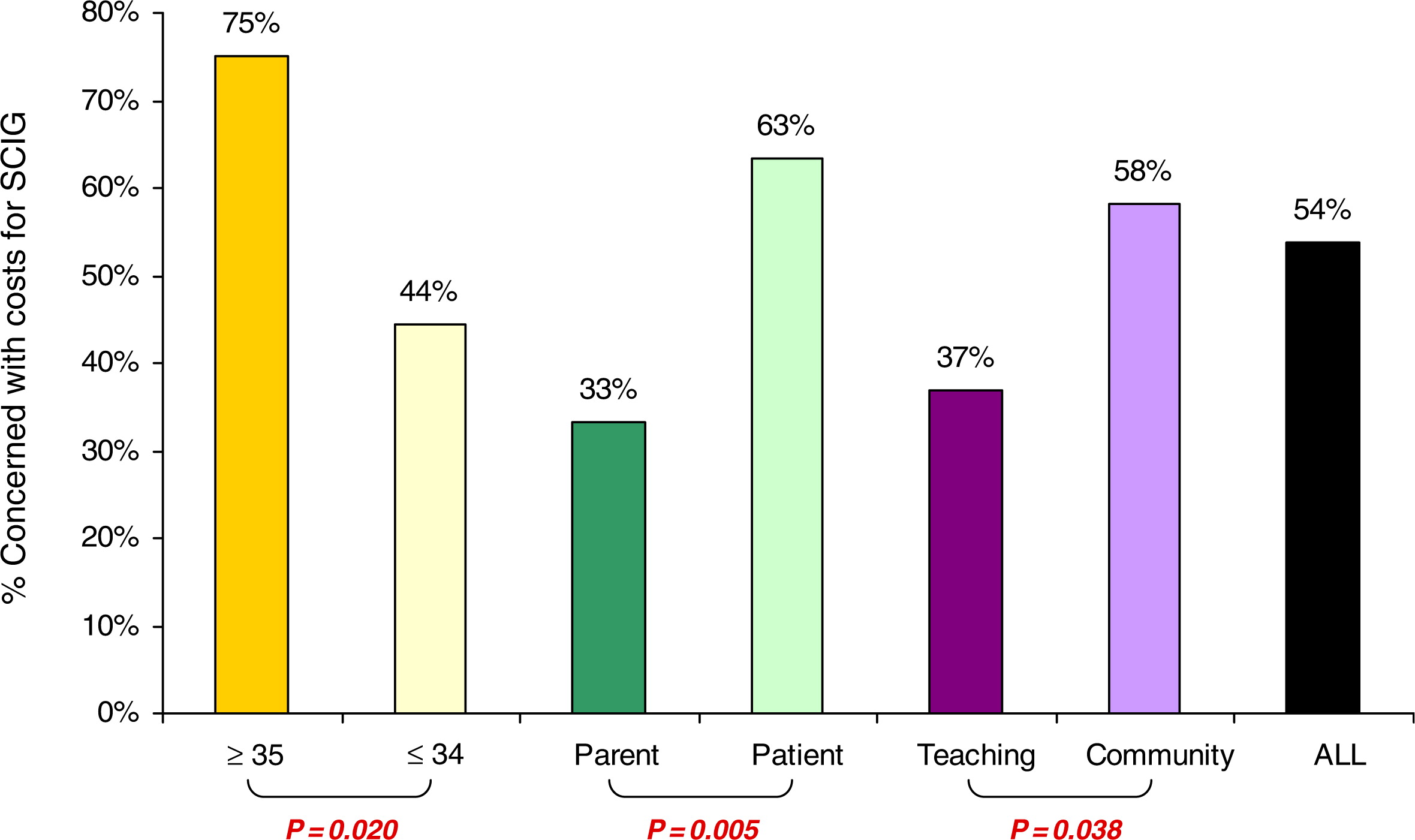
P = 0.017 and 0.049, respectively,
Figure 1). A similar trend was seen when patient concerns about cost were compared with parents; but this was not statistically significant (
Figure 1). Interestingly, patients 35 years and older favored home IVIG if a nurse was present to assist them (
P = 0.03). In addition, although all patients agreed that training of a family member in IVIG administration would not be essential to change to home therapy, significantly fewer patients over 34 years of age agreed with this (
P = 0.046,
Figure 1).