Physician–patient shared decision making in the treatment of primary immunodeficiency: an interview-based survey of immunologists
Abstract
Introduction
Methods
Qualitative approach and research paradigm
Concepts used in developing the questionnaire
Dual Process
Uncertainty
Bias (and nudging)
Power imbalance
Traits (physician and patient)
Experience
Trust
Organizational
Policy
Rules and time
Coordination
Feedback
Colleagues
Reimbursement
Reflexivity
Participants and interview
Ethics
Results
Participants
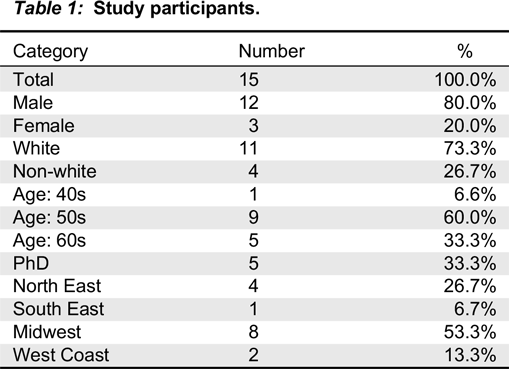
Interview development
Interview findings
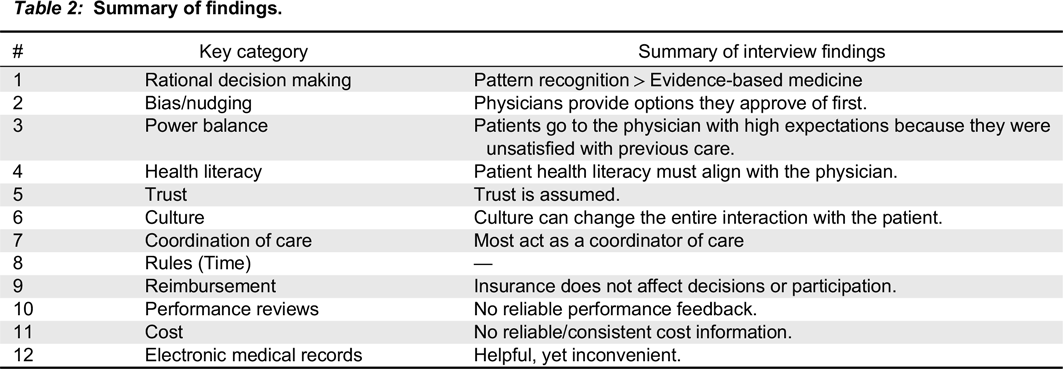
Long diagnosis period
It varies from one region to another. In rural areas, yes. In major cities like NY, Toronto, LA, no. The moment you start lumping up different regions, you are not going to solve well what is behind it. I would say that if there is a delay of treatment I don’t see very much of this in our place. We need to study it more carefully. Nothing is simple. If you say a delay of diagnosis in [IA], I say no. if you say overall PID, possibly yes; not because only knowledge, but progress of the field. We identified [IA] that had infections for a long time that never had PID, but even if we did, I doubt we would have managed to label that way because our diagnostic tools are much better today. Also do you include autoimmunity in that category? Or cancer? The delay in diagnosis for PID is hard for me to accept 7 years. It was a survey, not a study. Part of pushing the enzyme issue. We all support it, but it is not studied. (P07)
Often times that’s because people don’t get in to see a specialist in primary immunodeficiency. I think that many patients who talk about these diagnostic delays will talk about, “Oh, it was this breath of fresh air when I got to see Dr. X.” Well Dr. X was just somebody who’s trained in this process of true pattern recognition, and has the 90 minutes to go through and do it, as opposed to community-based allergist that’s trying to fit this into an otherwise 20-patient workday. I also think that … That’s one reason for diagnostic delay, getting to the true specialist. The other is that some of these diagnoses do evolve over time, so that when you see someone at point A, the laboratory tests may not necessarily have caught up to what their history is, and some of that evolution does happen over time as well. So those two reasons. (P12)
I think, again, another kudos to the advocacy networks like Jeffrey Modell Foundation, Immune Deficiency Foundation. I mean, they get the word out to inform people and put placards up in airports, community areas that have a lot of traffic, to tell people about these conditions. As physicians, we don’t do a good job of that. So people are becoming more aware. But I think there’s still an awareness gap. I do think patients are coming, and I’ve seen it frequently. I mean, I just saw a patient who’s 67 years’ old who actually makes absolutely no antibody whatsoever; none whatsoever; makes no antibody-producing cells; was actually diagnosed 35 years ago and put on IgG replacement therapy, but then stopped due to faulty information, and has been on antibiotics time, and time, and time again, essentially, continually for 35 years. [How did they get to you?] She ended up seeing a very good colleague in the community who was like, “Whoa, you’ve got a big problem. You need to go to the center where they’re used to taking care of this.” So they came over. Gave her her first infusion of IgG, and bridged her with some antibiotics because she was sick, and then hopefully she’s going to do well. (P11)
Rational/slow thinking
I think that evidence-based medicine is, it’s there to provide some type of quality control and some type of guidance towards where we want to move. But we always have to understand where evidence-based medicine comes from. (P08)I have a general idea of how much gamma globulin I want to give somebody based on data but I can tell you that individual patients don’t respond the way the median response in a paper, so I can tell you that lots of people will do fine with a gamma globulin replacement of about let’s say 500 milligrams per kilogram per month and there are other patients with exactly the same kind of characteristics that may do fine with 400 and others who may need 1000. (P01)
Bias and nudging
I guess it’s listening to the patient and offering things in a fair and objective way. I think those are the most important factors. So if I can understand someone and lay things out fairly and help guide them, ‘cause I’m sure that I’m biased with what I think is right, but I don’t want to ever force someone to do something, because I think it’ll backfire. I’d rather encourage them and tell them why I think they should do something and have them agree and buy in, otherwise you don’t get the compliance and outcome you want. (P03)
As a pediatrician, dealing with the issues, that what you did not want to have is a scenario set up where anyone would perceive blame. So you don’t want the physician to be blamed for whatever is done, you don’t want a parent, either parent, to be blamed or feel blame. And so, what’s done is a collective decision making. Now there’s sometimes when the cost of your medical knowledge, you believe the decision should be in a certain direction. And if the parent wants, or the patient wants, are counter to that, you try to use, for want of better words, savvy psychology to help them understand why that may be a preferred route to what they’re thinking. Many people have mixed perceptions of things or read testimonies that are incorrect because someone has a grudge on one or the other. And so what you do is you lay out the perspectives. If they’re equally good, you don’t add any bias to it. (P02)
I’m very sensitive to this issue. I would say if I’m unbiased, no way. Everyone has their own ideas and experiences. Everyone is biased one way or another. We try to present in an unbiased way. It is just human nature. You just try and find the best way. I found that you get better over time in dealing with being challenged by patients and ideas and being open to new ideas. Experience gives you flexibility. I think it also has to do with egos as well. I am definitely better than 25 years ago. (P07)
Findings related to SIT and agency theory
Power balance
I think when they come here to us, already they have the highest expectations because either they can come here because they didn’t get the satisfactory treatment or approach elsewhere or they came here for unique things we do for newborn screening, or they’re just referred to us because the other part didn’t know what to do. (P15)
I would, usually, emphasise to the patient that in order to progress along this path of diagnosis to treatment, we need to do x, y, z. In order to understand the problem more clearly. There isn’t usually very much of a discussion about the pro’s and con’s, and risk, benefit, cost, et cetera. Most of the time, during that process. There are circumstances where, specifically, cost will become an issue. (P04)I mean sometimes somebody would say, “I want to try facilitated subcutaneous, because I heard about it.” That’s fine. That’s great. If their insurance will let them have it, we’ll get that for them. If somebody’s on IV and wants to go to subcu, that’s great too. (P12)
That’s an area that I’ve thought a lot about and kind of very conscious about, so I never address an adult patient of mine by their first name, no matter, I’ve known people for 30 years, I’ve gone to their kids’ weddings or whatever, I never ever ever address an adult patient of mine except as Mr. or Mrs. or Ms. and I do it because I think I need to maintain a certain degree of professional distance, maybe part of that is to protect myself but part of that is there are certain times in a doctor–patient relationship when you have to say to somebody, you have to give somebody bad news or you have to say to them “I know this is what you want to do but I think this is really wrong”. (P01)
Health literacy
So, there are people who have read x, y, and z on the ‘net and they may consider themselves to be health literate but they’re getting a lot of misinformation which can really cause problems. Because now you’ve gotta sort of undo what they’ve read and redirect them to what the actual reality is of the treatment. (P05)I think it’s helpful; in general, I do. I think the internet is a great resource for people. Problem is when they go to chat rooms and hear weird things from different people, it means nothing. You have to use reputable sites, so we actually give out our list of reputable sites for information on immune deficiency for patients, so they can read at their leisure and look things up. (P03)
I feel like those patients feel like they have a plan. That they can go and find people … I think humans, by nature, are herd animals. When they find like-minded people that are going through the same thing, and they don’t feel so isolated, then you don’t have the anxiety, depression, and all of the other issues related to treating a child with a chronic disease or having a child with a chronic disease. They can find like-minded people that have been through the same things and can help them with the day-to-day things that I don’t necessarily have advice for because I don’t live with it every day. (P13)
Findings related to traits
I think it’s really good, but I always keep telling them, take everything with a grain of salt, because each patient is really their own individual disease process. We look at each patient as their own individual experiment. And so what’s solely true for this other patient, and because you think the symptoms are the same, there only telling you a fraction of the process. And while that fraction may seem to match up, may not be the direction towards getting the right answer type of thing. So it’s very helpful, but it also helps the patients to get guided to the right physicians to help with the thing. (P02)
Trust
I think for my personality, and again, coming as a pediatrician, my patients call me by my first name, and because their parents call me by my first name, and so some of the kids will start calling me by my first name. And I’ve never been pretentious about that issue, and never tried to correct people on that. And so I think I come across, for myself, less intimidating, and so there’s a trust that builds up because I’m not trying to snow them, I’m not trying to pull the wool over their eyes, I’m not trying to intimidate them, and so I think happens is because they realize I’m well educated, many actually want me to help more in the decision, not realizing that I’m psychologically trying to help them in the process. They’re actually wanting more of my input. Even physicians I’ve dealt with. (P02)
I use their noncompliance, or the dishonesty as evidence in a very professional and transparent way, as to why I feel the way I feel, and then I’m very clear that I’m going to document that this is my recommendation, and they don’t take it, that they’re going against medical advice. That it’s their choice to go against medical advice, and if their child gets sick, there are consequences for that. I’m very clear about that and because when you set that expectation, in a patient that has good intentions, they will work with you. They will understand that they have been at fault, but if their intentions are good, and there’s no secondary gain, then 9 times out of 10, they will actually comply with you. When there is secondary gain, then I have protected myself, and told them what the consequences would be and set expectations. When they fail to meet my trust again, then I can take recourse to protect the child. [What would be the secondary gain?] “My child is my proxy-ish” kind of thing. I don’t know. Parents like the attention. That’s what we consider secondary gain. For everybody, I don’t know what that would be. (P13)
Culture/family
And so, for example, I’ve dealt with individuals from the Middle East, and so one of the things we learn as being a pediatrician, obviously, is to make contact with the individual. And so shake their hand, depending on the severity of the process and things that are going on. Perhaps hold their hand a while. Usually a mother, or a female, or the child type of thing for that. Especially a child. Have them sit in your lap, you know younger children, sit in your lap while you’re doing all this and hold them, so that you reach out. Some of the Middle Eastern cultures, you know, it’s very offensive for a male to touch a female, for example. And so you have to learn to know that you can’t use the same context for connecting. [Is that something you just learned over time?] Part of that was learning, but part of it was also when having interpreters and others that would help explain the cultural differences on there. In the U.S., African American tends to be more concerned about … So the African American, there is more distrust of the healthcare system. With good reason for a variety of the things that have occurred. And so you have to develop that trust from the very beginning, and honesty from the very beginning on there. And establish the fact that you recognize they’re African American. You point out there’s specific items and issues that are more unique towards African Americans than to Caucasians. And you, again, you create these, not boundaries, but openness to that where you can generate that trust, you know?! That “I’m not gonna be perpetrating on you things that are against your will, or that otherwise would be harmful. That color of your skin is not a barrier to being able to achieve the healthcare that you need.” Hispanics, different cultural things. (P12)
Hmong believe in a lot of tribalism. From Southeast Asia. Gypsies. Gypsies are very interesting … they’re always very distrustful of anyone because they think everyone’s always out to get them. And usually, with the gypsies, you are in a room of ten people, because they bring in the elders and everybody to all that. (P12)
Findings related to organizational context
Coordination of care
… I’m a pediatrician so there I’m much more of a coordinator, but the continuity doesn’t change, I think that’s one of the really big things that we offer in our clinic is from the time that I started and I was the junior person with two people in the clinic, we decided the way we were going to operate is that each of us was just going to take new patients, we would split them up or whatever way it worked but once you had seen the patient that patient was just going to be your patient and whenever they came into the clinic for a follow-up, if it was my patient I would see that patient, if they were admitted to the hospital, I would go to see them in the hospital. (P01)
…when it comes down to the therapy that I’m proposing; for example, immunoglobulin, or gamma interferon, or Rituxan, or antibio, or anything else, naturally, I’m going to coordinate and make that happen. I’m going to make sure it happens. If it’s a person who has a gastrointestinal condition, it’s not me. I’m not a gastrointestinal doctor. So I’m going to help them see that other doctor. So I’m going to coordinate on one hand, and do continuity on the other. Mine’s continuity; the things that I have suggested. If I have to send them to a rheumatologist, or to a pulmonary doctor, their pulmonary hypertension, or their hematology, I have to send that person over to the other quadrant for all of that. (P06)
Rules related to time
…in the ideal world, as I think you’re eluding to, what we’d have are primary care physicians that would be set up in a scenario where they would not be having to see 50 patients a day, but be able to see 25 patients a day. (P02)
Reimbursement
Yeah, I don’t win all the time. The big issue is that the insurance companies out there are not aware of what the evidence shows in the literature. Most of the time what they do is they rely on “physician review” and these physicians have absolutely no understanding of any particular field. … Most of those cases get approved after a lot of fighting. Even then sometimes insurance companies are like no we’re not doing this. (P14)
Findings related to feedback
Performance reviews
I don’t think I’m an intimidating presence. That’s not my style at all. I mean, I will tell somebody if I think they’re making a bad decision, certainly. But, I think because I really do try to make this a discussion, you know, a lot of feedback from the patient. I think most of the time they tell me they feel much more comfortable with the diagnosis, much more comfortable with the treatment plan, because we’ve had this conversation. And I do get a lot of referrals from people who come in and see me specifically. So, I think they feel like they’ve talked to somebody who’s got a lot of experience, a lot of expertise. (P05)
[Is there any systematic feedback?] There’s not any systematic feedback. Usually … I’ve been fortunate enough that no one has lodged a complaint against me where administration and patient advocacy have had to get involved. I have gotten feedback when patients have said nice things to me, nice things about me. I don’t get consistent feedback and I think the only consistent mechanism, by way that the hospital has the patients rate us is through the Press Ganey surveys. After that incident where I had that horrible review online, I have stopped googling myself. I’m a caretaker, I’m a feeler. I take those things really personally because I don’t want other people, and other patients, who I take care of to read something like that and then lose their trust in me because of something they read on the internet. These last two years is a perfect example of seeing things on the internet that aren’t really true or not the full side of the story. They rely on that too much… (P13)
Cost
[In the 300 patients that you have, is there any way that someone looks at the cost of treating them over the course of the year and the outcomes? Is there any way that someone says, “Wow, here’s the 300th.” And why not?] You know, what I’ll say is that there’s no systematic way that is intentionally done for my individual patients that gives me feedback. I do hear from payers, from time to time, to say, “This patient on X asthma therapy,” again, not talking about immunodeficiency, but kind of an easier disorder to talk about because there are just much more metrics in place to track quality and so forth, “you might consider stepping them down from drug X to drug Y.” And I think that’s based on guidelines. It’s also based on cost. For sure. There’s no doubt. I mean, let’s face it. We all get that. So there are some of those things. There’s also, even within our own system, like, our health plan covers women and children who are of lower income. It’s a medical assistance, Medicaid-based payer. So we will get feedback on use of expensive medications and lower-cost alternatives. There are some of those things out there, but there is nothing that tracks, “Yeah, here’s your 300 patients. 250 of them are optimally treated based on these outcome measures. You’re providing as cost-effective care as you can based on the complexity level of the patient, available resources, et cetera” (P11)
Electronic medical records
[Are electronic data records a good thing?] Oh yeah. I think so because I can read everybody’s notes. [Any downside to electronic records?] Sometimes, you need to be careful what you really need to write. For example, some of the patients, but we do sometimes research testing, of course after getting consent, it’s not … But some other sub specialist that’s taking care of the same patient for another thing, they … If parents tell that to that doctor and they put it down, that’s a problem because research data cannot be in the medical records for clinical care. They happen to me a couple of times which is difficult because then you have to addend. But, it’s not good to disappear completely. If the medical records wants to go back and look at it, they can see it. It’s going to go to the record that the other provider will see, but it will be in the records see, there is no way you can correct it or get rid of it. [In general terms of caring for the patient] It makes it very easy. (P15)
Discussion
Acknowledgements
REFERENCES
Appendix A Interview guide
Information & Authors
Information
Published In

History
Copyright
Authors
Competing Interests
Funding Information
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
There are no citations for this item
View Options
View options
Get Access
Login options
Check if you access through your login credentials or your institution to get full access on this article.


