Introduction
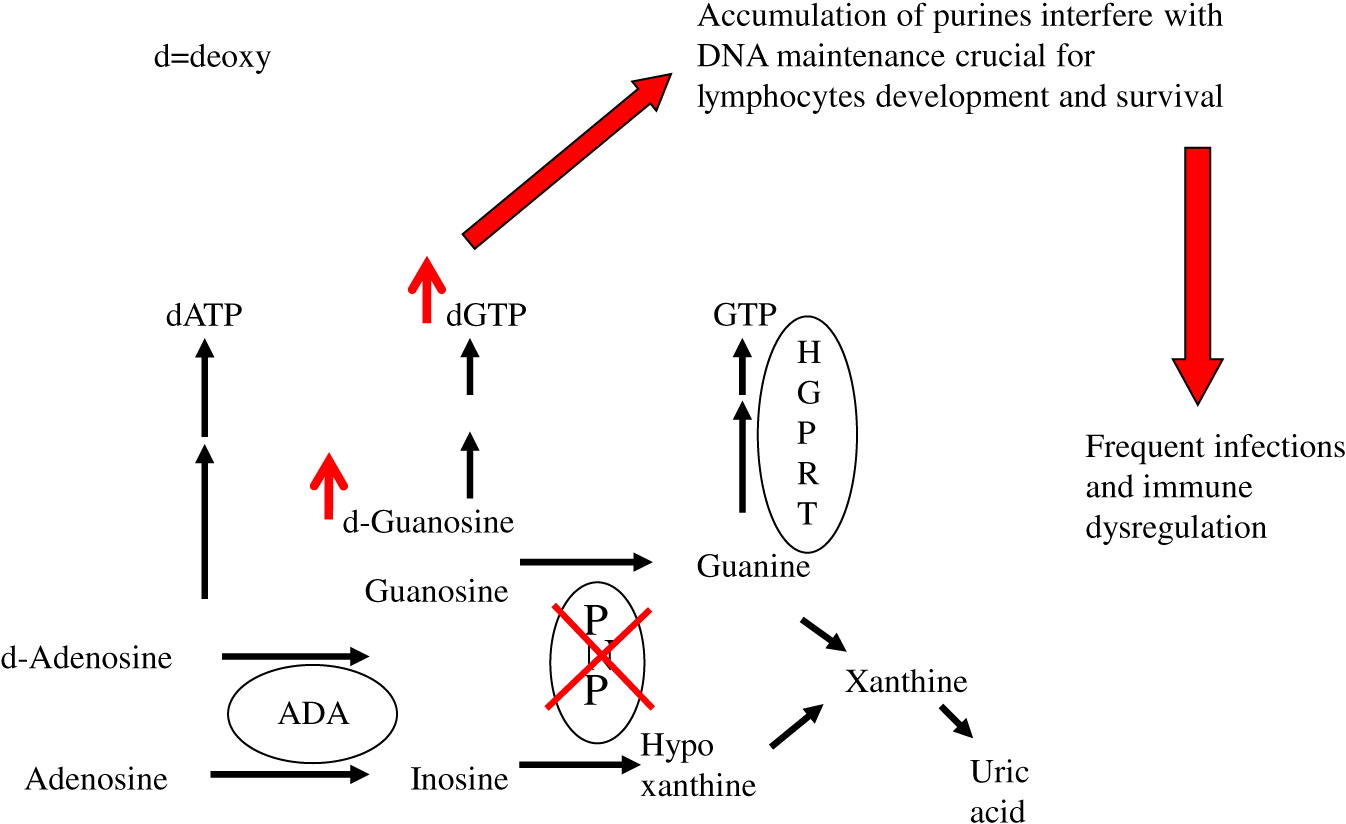
Purine nucleoside phosphorylase (PNP) is an important enzyme in the phosphorylation of guanosine and deoxyguanosine to inosine and hypoxanthine, which can eventually be excreted as uric acid (
Figure 1). Alternatively, deoxyguanosine can be phosphorylated by deoxyguanosine kinase to dGTP. Inherited defects in PNP, resulting in <5% enzyme activity, cause an accumulation of PNP substrates. There is also depletion of PNP products, often leading to a reduction in hypoxanthine and uric acid (
Cohen et al. 2000). The impaired purine metabolism leads to enhanced thymocyte apoptosis, reduced secretion of cytokines by immune cells, and impaired proliferation of peripheral T cells in response to mitogens and allogeneic cells (
Arpaia et al. 2000;
Grunebaum et al. 2004;
Yu et al. 2009;
Papinazath et al. 2011). The T cell dysfunction results in increased susceptibility to infections, autoimmunity, and malignancy (
Watson et al. 1981;
Delicou et al. 2007). In some patients, defects in NK- and B-lymphocytes were also reported, further increasing patients’ risk for infections (
Somech et al. 2013). PNP is ubiquitously expressed, suggesting that PNP deficiency might also affect non-lymphoid tissues. Indeed, dysplastic bone marrow with increased sensitivity of bone marrow cells to irradiation has previously been reported (
Dror et al. 2004). In addition, more than 50% of patients with PNP deficiency exhibit diverse non-infectious neurological dysfunction that often precede the immunological abnormalities, and persist even after correction of the immune deficiency (
Baguette et al. 2002;
Tabarki et al. 2003;
Ozkinay et al. 2007;
Grunebaum et al. 2013).
Limited access to tissues from patients and ethical considerations have impeded the ability to study mechanisms responsible for the abnormalities observed in PNP-deficient patients. A PNP-deficient (PNP−/−) mouse model that recapitulated many of the human manifestations has allowed overcoming some of these limitations (
Arpaia et al. 2000). PNP−/− mice demonstrated decreased ability to remain on a revolving rota-rod, an assay used to assess ataxia in mice, as well as smaller cerebellum, reduced number of cerebellar Purkinje cells and increased apoptosis of neurons, in comparison to healthy PNP-proficient littermate mice (
Mansouri et al. 2012). Moreover, repeated injections with PNP fused to the HIV TAT protein transduction domain (
Toro and Grunebaum 2006) corrected the PNP deficiency and prevented the occurrence of these abnormalities (
Mansouri et al. 2012). However, the precise mechanism for the neuronal apoptosis was not determined, nor was it clear whether the immune abnormalities observed in the PNP−/− mice contributed to the phenotype.
Induced pluripotent stem cells (iPSC) have recently been used to better understand the pathogenesis of diverse neurological disorders (
Barral and Kurian 2016). Using lymphocytes, fibroblasts, skin cells and other sources, which can be de-differentiated into iPSC and then matured into specific lineages, iPSC have been shown to be a robust tool in generating unlimited amounts of cells that simulate many stages of human neuronal development (
Mertens et al. 2016).
Accordingly, we hypothesized that studying neurons cells differentiated from the iPSC of PNP-deficient patients would enable better appreciation of the role that perturbed PNP metabolism has on neurons, as well as treatments for this condition. Here we present preliminary results demonstrating the utility of such cells in evaluating the effects of PNP deficiency on neuron cell development.
Methods
EBV-transformed lymphocytes from PNP-deficient patient and healthy controls were used to generate iPSC lines by the Canadian Center for Regenerative Medicine (Toronto, ON) with Sendai viruses, as described previously (
Patel and Yang 2010). PNP activity of the cells was assessed by measuring conversion of [8-14C]inosine (50 mCi/mmol; Moravek Biochemicals, Brea, CA, USA) to hypoxanthine using cellulose TLC, as described previously (
Toro et al. 2006).
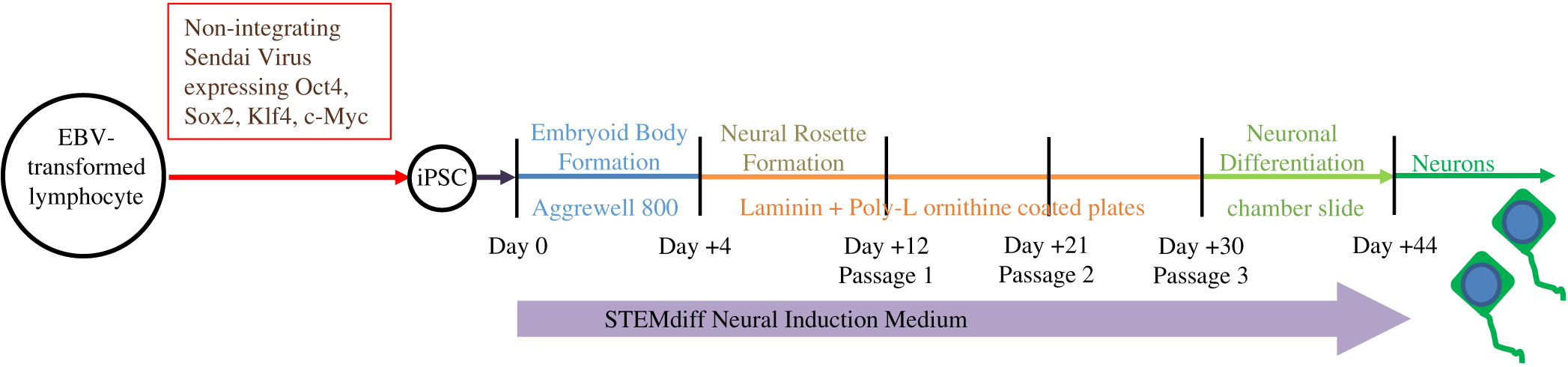
As described in
Figure 2, iPSC were differentiated through the formation of embryoid bodies by dissociating iPSC and plating 2 × 10
6 cells/well in Aggrewell 800 plates (STEMCELL Technologies, Vancouver, BC). After 4 days, embryoid bodies were dissociated and plated onto Laminin and Poly-
l-ornithine coated 6-well plates for an additional 7 days. Following formation of neural rosettes, rosettes were detached using STEMdiff Neural Rosette Selection Reagent (STEMCELL) and plated onto Laminin and Poly-
l-ornithine coated plates at a minimum density of 2 × 10
6 cells/well. The neural progenitor cells were then cultured to passage 3 and differentiated into neurons by plating 1 × 10
5 cells/well into chamber-slides. STEMdiff Neural Induction Medium (STEMCELL), which is serum free and devoid of PNP activity, was utilized throughout the entire differentiation.
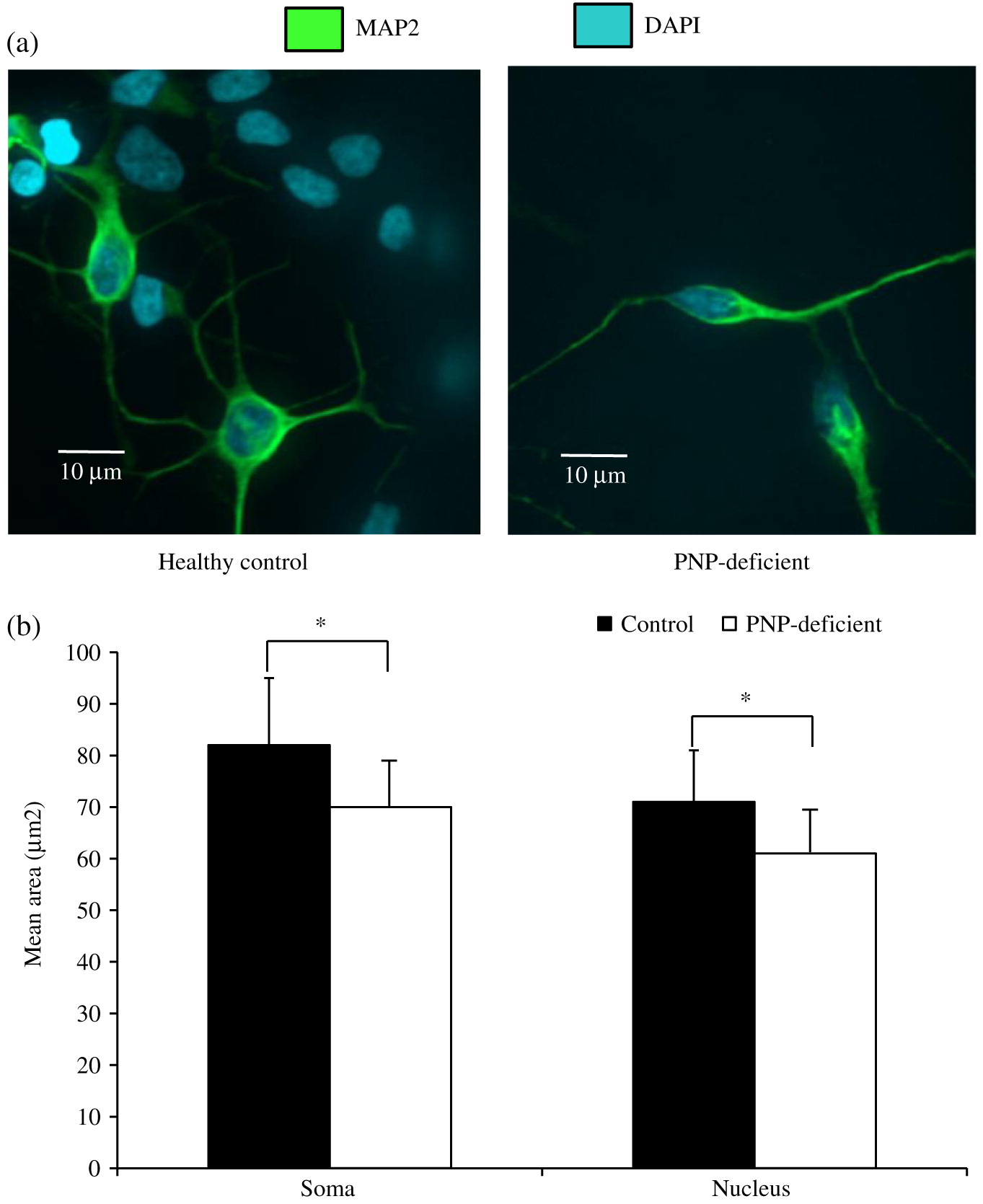
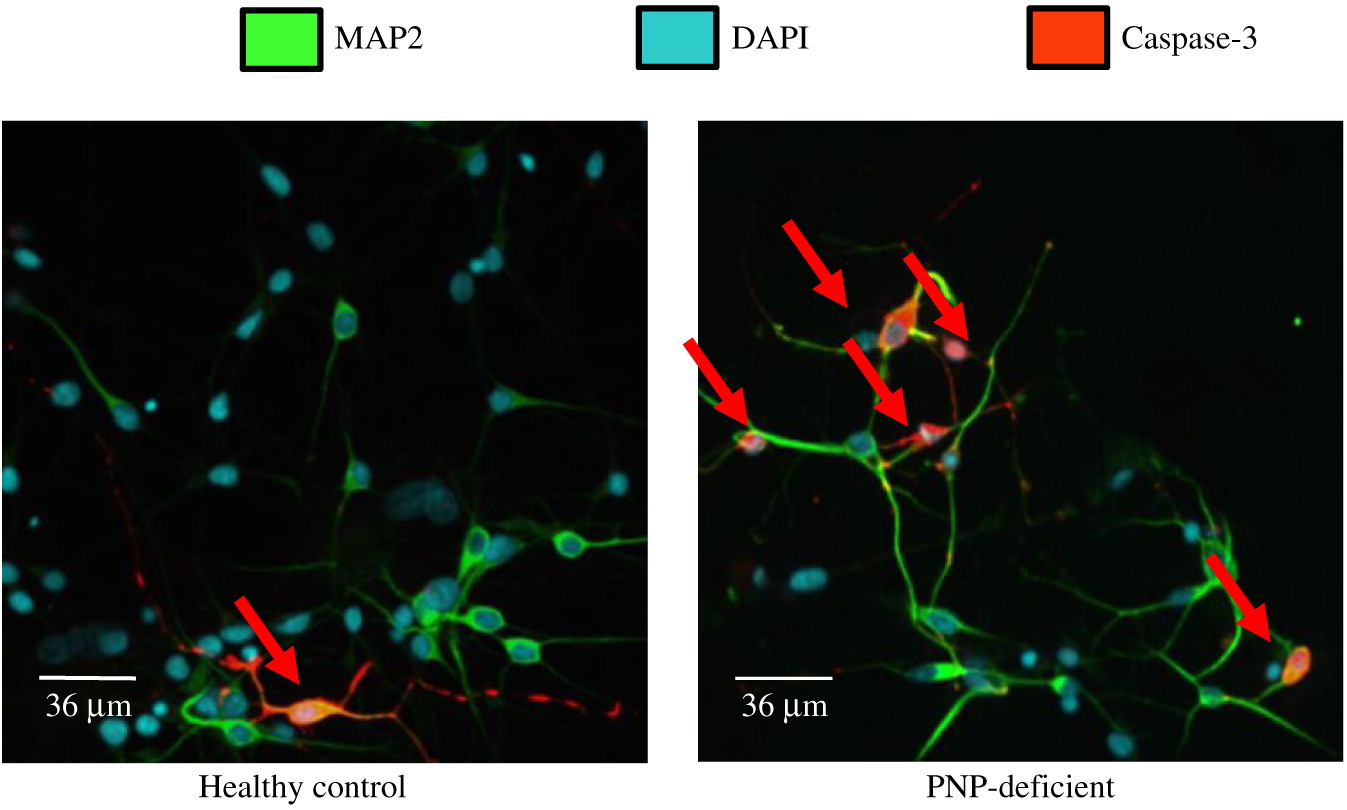
Neuronal cell soma and nuclei were stained with anti-MAP2 antibody (Abcam, Cambridge, MA, USA) and DAPI (ThermoFisher, Waltham, MA, USA), respectively. Soma and nuclei size analysis was performed using Improvision Volocity software (PerkinElmer, Woodbridge, ON) on images from more than 150 randomly selected fields from 30 cover slips over 3 independent biological replicates of neuronal differentiation per iPSC. Team members unaware to the PNP status of the cells performed these measurements. Apoptosis was detected by expression of cleaved Caspase-3 (Abcam) among cells mounted on slides. Statistical significance was determined using unpaired equal variance t test.
Discussion
Studying the neurological effects of impaired PNP function has been challenging as many patients suffer from infections, autoimmunity, and neurological abnormalities, which might impact their growth and development with secondary effects on brain development. Moreover, obtaining brain tissue is ethically unacceptable unless patients are undergoing evaluations for extensive neurological damage or succumb to their disease. As an alternative, we and other researchers have used animal models to better understand the effects of inherited purine defects on brain development, although differences in brain formation and function between mice and human limit the ability to draw conclusions relevant to humans (
Jinnah et al. 1990;
Mansouri et al. 2012;
Sauer et al. 2017). Accordingly, the availability of patient-derived iPSC, which can be continuously propagated and directly differentiated to simulate human mature neuronal cells is an important tool. Indeed, in recent years the number of inherited neurological diseases that are modeled by iPSC has grown remarkably (
Andrade et al. 2012;
Chailangkarn et al. 2016;
Allende et al. 2018).
Some barriers to reprogramming of differentiated cells into iPSC have been identified, such as senescence, expression of growth suppressor genes or increased sensitivity of the cells’ DNA to various noxious damage (
Andrade et al. 2012). Although PNP is a house keeping enzyme important also for DNA and RNA formation and repair, PNP seems to be dispensable for generating pluripotent stem cells. This finding is in agreement with the development of PNP−/− mice embryonic stem cells, evident by the birth of PNP−/− pups from mating PNP−/− female and male mice (E. Grunebaum, unpublished data).
Our results show that while PNP deficiency does not prevent generation of neuronal cells, the PNP-deficient cells have impaired morphology and survival. The relevance of PNP-deficient iPSC derived neuronal cells’ small soma and nuclei size to the neurological and histological abnormalities observed in PNP-deficient patients and mice is still not clear. Although needed to be replicated in neuronal cells derived from other PNP-deficient patients, our findings are similar to those reported among iPSC derived from patients with Rett syndrome, a severe neurological disease caused by MECP2 mutations (
Cheung et al. 2011), supporting the validity of our data. Moreover, it will be important to determine whether PNP deficiency also distorts the morphology of iPSC that can now be developed using new techniques into cerebellum- and purkinje cells (
Watson et al. 2018), as observed in PNP−/− mice (
Mansouri et al. 2012).
Interestingly, we found increased apoptosis in PNP-deficient neuronal cells, similar to the enhanced cells’ death already identified in PNP−/− mice thymocytes and cerebellar Purkinje cells (
Papinazath et al. 2011;
Mansouri et al. 2012). Although the spontaneous apoptosis of PNP-deficient neuronal cells was only about 2 fold higher than in normal cells, this rate is not much different than the 3–4 fold apoptosis increase in Cockayne- and Williams-syndrome iPSC derived cells, respectively (
Andrade et al. 2012;
Chailangkarn et al. 2016). Hence our data suggests that in progressive neurological diseases of childhood, such as PNP deficiency, even a small increase in apoptosis might be clinically relevant. The precise mechanism(s) leading to the apoptosis in PNP-deficient neuronal cells has not been established yet, however we have previously shown that the apoptosis in PNP deficiency is typically initiated in the mitochondria, and can occur in response to different conditions. These include exposure to deoxyguanosine, dexamethasone, or irradiation (
Dror et al. 2004;
Papinazath et al. 2011). Hence, it will be important to assess susceptibility of PNP-deficient neuronal cells to these factors as they might be generated or utilized when treating PNP-deficient patients for infections, autoimmunity, malignancy, or performing hematopoietic stem cell (HSC) transplantations. Better appreciation of the causes of PNP-deficient neuronal cell death might prompt avoidance of such compromising situations.
The availability of iPSC derived neuronal cells will also provide an opportunity to assess the effects of different treatment options on the neurological abnormalities associated with PNP deficiency. Transfusions of normal red blood cells rich in PNP (
Rich et al. 1980), transplants with normal allogeneic (
Yeates et al. 2017) or gene corrected autologous (
Liao et al. 2008) HSC transplantations or frequent injections of native (
Toro and Grunebaum 2006) or PEGylated (E. Grunebaum, unpublished data) PNP, have all been investigated for the management of PNP deficiency. However, all these treatments are based on “cross-correction” of the toxic purine metabolites i.e., exit of nucleotides from the cytoplasm of cells in accordance to the purine concentration gradients. An alternative approach might be needed for the neurological deficits, such as delivery of PNP across the blood brain barrier and into the cells with the HIV-TAT protein transduction domain (
Toro and Grunebaum 2006) or a modified diphtheria toxin (
Auger et al. 2015). Finally, an exciting recent development in the use of iPSC is the creation of 3 dimensional brain organoids, which might provide a robust pre-clinical tool for assessing management strategies for the brain abnormalities prior to initiation of patients’ trials.